INTRODUCTION
METHODS
Patients
Surgical procedures
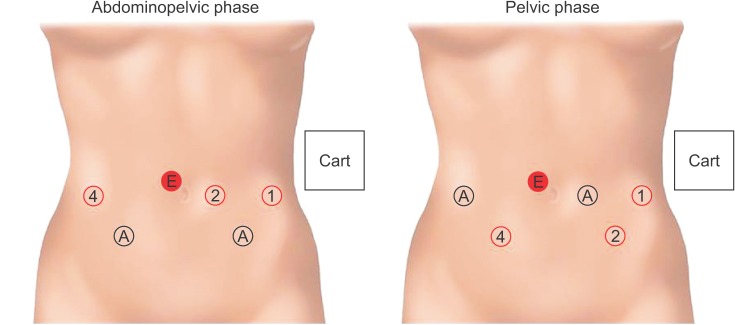 | Fig. 1Port positioning and instrument installation for the abdominopelvic phase and pelvic phase using a da Vinci Xi system (Intuitive Surgical Inc., Sunnyvale, CA, USA). All ports are 8 mm, except for the right lateral port used for the Smart stapler and Hemolok (TeleFlex, Westmeath, Ireland). The 8mm endoscope port is placed about 1 cm right and cephalad to the umbilicus. The remaining 3 horizontal ports are then placed on the umbilical line, i.e., 2 lateral ports 2 cm from the midclavicular line, and a left medial port 6–8 cm from the lateral port. Right and left quadrant ports are placed at the McBurney's point and counterMcBurney's point respectively. Instruments used are as follows: tipup fenestrated grasper ①, Maryland bipolar grasper ②, and monopolar curved scissors ④ for the abdominopelvic phase; Maryland bipolar grasper ①, tipup fenestrated grasper ②, monopolar curved scissors ④ for the pelvic phase. E, endoscope port; A, assistant port. |
Postoperative follow-up and evaluations
Statistical analysis
RESULTS
Patient and tumor characteristics
Table 1
Physical and tumor characteristics of the patients
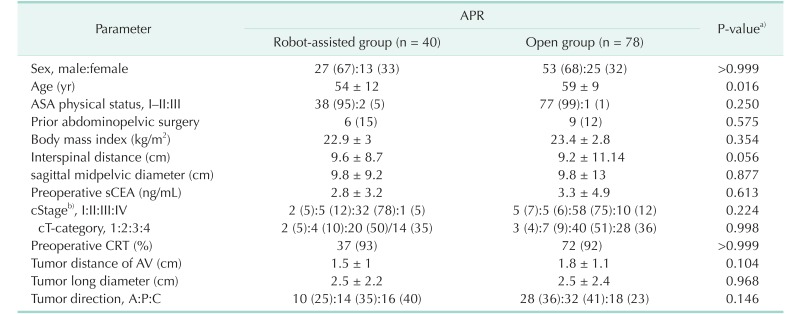
Values are presented as number (%) or mean ± standard deviation.
APR, abdominoperineal resection; ASA, American Society of Anesthesiologists; sCEA, serum carcinoembryonic antigen; cStage/cT, clinical American Joint Committee on Cancer stage/T category; CRT, chemoradiotherapy; AV, anal verge; A:P:C, anterior:posterior:combined.
a)All parameters were compared using Fisher exact test with 2-sided verification or Pearson chisquare test and an unpaired t-test. b)Cancer staging according to the American Joint Committee on Cancer (8th ed., 2017). Preoperative clinical staging was determined by CT and MRI.
Operative elements and measures
Table 2
Operative and postoperative outcomes
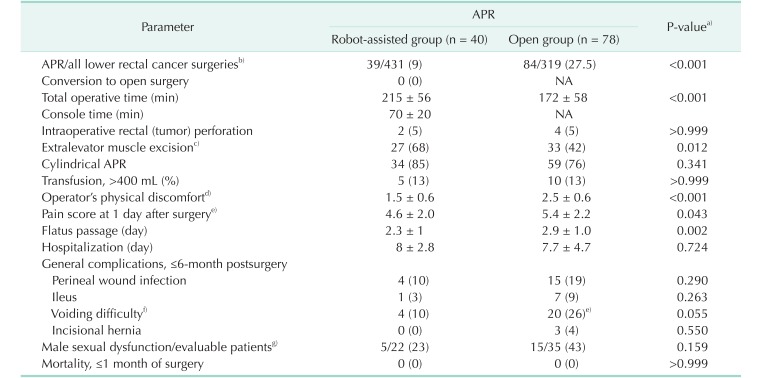
Values are presented as number (%) or mean ± standard deviation.
APR, abdominoperineal resection; NA, not applicable.
a)All parameters were compared using Fisher exact test with 2-sided verification and an unpaired t-test. b)The number of lower rectal adenocarcinoma patients (tumor located ≤6 cm from the anal verge) with R0 resections during the study period. c)APR with en bloc excision of more than either one or both side(s) of the puborectalis and/or pubococcygeus muscles. d)Assessed by the surgeon using a visual analog scale (a modification of Lawson's: scales 0–5) immediately after the operation [15]. e)According to the patient's subjective evaluation of pain on a visual analogue scale, 0 (none) to 10 (agonizing). f)All patients recovered by supportive management within 3 months of surgery, except for one patient in each group who needed intermittent catheterization. g)Assessed regarding erectile firmness and ejaculatory frequency, presented by grades using a visual analog scale, i.e., none-mild (0–1), moderate (2–3), and severe (4–5). Male sexual dysfunction: ≤65-year-old patients with moderate and severe grades of either erectile or ejaculatory potency.
Postoperative courses and complications
Pathological features related with oncological outcome
Table 3
Pathological features
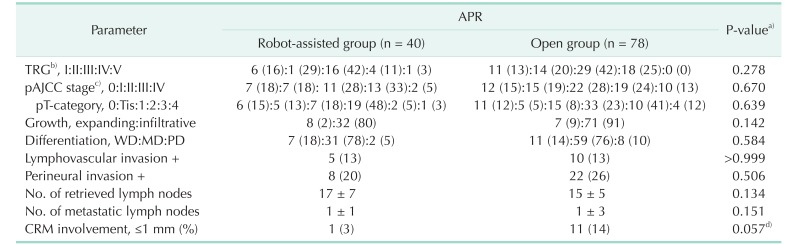
Values are presented as number (%) or mean ± standard deviation.
APR, abdominoperineal resection; TRG, tumor regression grade; pStage/pT, pathological American Joint Committee on Cancer (AJCC) stage/T category; WD:MD:PD, well:moderately:poorly differentiated; CRM, circumferential resection margin.
a)All parameters were compared using Fisher exact test with 2-sided verification or Pearson chi-square test and an unpaired t-test. b)TRG 1, 2, 3, 4, and 5 defined as complete, near total, moderate, minimal and no responses, respectively. c)Cancer staging according to the American Joint Committee on Cancer (8th ed., 2017). d)P = 0.042 in 1-sided verification.
Table 4
Parameters associated with positivity of the circumferential resection margin
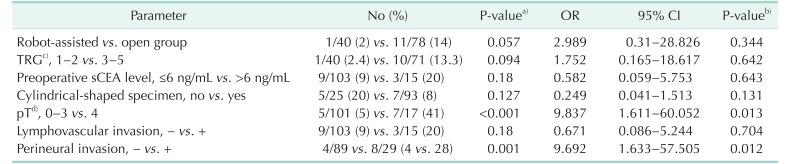
OR, odds ratio; CI, confidence interval; TRG, tumor regression grade after preoperative chemoradiotherapy; sCEA, serum carcinoembryonic antigen; pT, pathological American Joint Committee on Cancer T category.
a)Parameters were compared using Fisher exact test with 2-sided verification. b)Potential variables were verified by multivariate analysis using binary logistic regression. c)TRG 1, 2, 3, 4, and 5 defined as complete, near total, moderate, minimal and no responses, respectively. d)Cancer staging according to the American Joint Committee on Cancer (8th ed., 2017).
Recurrences and survival outcomes
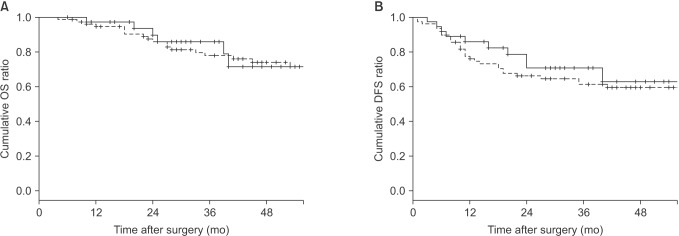 | Fig. 2The 2-year postoperative survival outcomes in 113 patients (robot-assisted APR group vs. open APR group, 36 vs. 77 patients) using the KaplanMeier method with the logrank test. Robot-assisted APR group vs. open APR group: 2-year OS, 85.9% vs. 82.9%, P = 0.819; 2-year DFS, 70.7% vs. 67.6%, P = 0.487. APR, abdominoperineal resection; OS, overall survival; DFS, diseasefree survival. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download