Abstract
Purpose
Nosocomial infections account for one of the most serious complications in hospitalized patients around the world. Surgical site infections have significant economic implications, and surgical antisepsis plays an important role in such processes.
Methods
With prior approval by the Institutional Review Board and informed consent, 10 volunteers were randomly assigned to 3 protocols on hand antisepsis: protocol A (chloroxylenol 3%), protocol B (benzalkonium chloride at 1%), and protocol C (ethyl alcohol 61%, 1% chlorhexidine gluconate). Smears from both hands were cultured after each hand pro tocol (t0) and at the end of suturing (t1). Colony forming units were counted (CFUs on blood agar dishes) with digital counting software (Open CFU). Friedman test was used to compare the mean values among the groups, and a Bonferroni correction was made to determine the dissimilar group, with a P = 0.015.
Nosocomial infections represent one of the major sources of morbidity and mortality in hospitalized patients around the world. Out of those infections, the most common one is the surgical site infection (SSI) [1]. According to the World Health Organization, in developing countries, the risk of infection related to health care practices is from 2 to 20 times higher than that in developed countries [2]. The unnoticed transmission of bacteria to patients' wounds during surgery may cause SSI. SSIs are one of the most common causes of health-care related infections for patients who undergo surgery [3].
The bacteria that cause SSI come from different sources in the operating room, including hands and surgical equipment. All the team members use sterile gloves to prevent spreading bacteria to the patient and vice versa. However, gloves may get punctured during surgery, thus it is important to have the hands germ-free. This can be achieved through surgical hand antisepsis right before wearing the gloves for the surgical procedure.
Simple hand washing, removes most of the transient skin bacteria, while hand antisepsis also inhibits the growth of resident bacteria, thus reducing the risk of SSI [345678]. The characteristics of an ideal antiseptic would be: immediate action, persistent (lasting several hours), cumulative activity (repeated exposure provides enhanced inhibition of the bacterial growth), broad spectrum of activity, and safe use by the operating room staff [910].
There are several strategies involved in hand surgical antisepsis: prior washing, washing technique, use of sponges, brushes, nail cleaning spatulas, duration of the antisepsis, and the antiseptic agent used.
Different terms are used to describe hand surgical antisepsis. Traditional antisepsis involves a brush, an antiseptic agent, and running water. Water-free antisepsis is one that involves alcohol-containing solutions.
The Association for Perioperative Practice (AfPP) recommends washing the hands before applying antisepsis. Washing should consist of using soap or an antimicrobial substance under running water (AfPP 2011). The purpose of hand washing is to remove dust and major gross contaminants. After that, the recommendation is to use plastic spatulas to clean the nails under running water. Finally, to perform either a traditional or water-free antisepsis [11].
Therefore, surgical hand antisepsis is one of the major factors in reducing the transmission of bacteria [12]. There are not many studies on surgical hand washing and its effect in the reduction of bacterial colonization [13].
The most widely used are ethanol, isopropanol and n-propanol. They are effective against gram positive and gram-negative bacteria, mycobacterium tuberculosis and many viruses, and fungi [14].
They are usually associated to polyvinylpyrrolidone to extend its duration, are effective against gram-positive, gram-negative bacteria, mycobacterium tuberculosis, and viruses [15].
It is a biguanide effective against gram-positive, gram-negative bacteria, lipophilic viruses and yeasts, nonsporicidal. It has a long-lasting action because it attaches to the horny layer of the skin [16].
The benzalkonium alkyl-chloride is the most used for hand antisepsis. It is bacteriostatic, fungistatic, and microbicidal at high concentrations. It has a higher activity against gram-positive bacteria than gram-negative and its activity is weak against mycobacterium and fungi. It has very good activity against lipophilic viruses. The U.S. Food and Drug Administration (FDA) has not fully approved its efficacy and safety as an antiseptic [8].
It is a phenolic compound with a halogenated substitute. Its action is NOT as quick as that of the chlorhexidine or iodophors and it exhibits a short residual activity. The FDA has not fully approved its efficacy and safety as an antiseptic [17].
Today, there is a large amount of antiseptics with various active ingredients whose properties have not been analyzed in our setting that could be very useful for hand washing and thus reduce the transmission rate of infections [181920].
In this work, 3 surgical hand antisepsis methods are compared in order to measure their effectiveness in reducing the bacterial colony forming units, as well as to evaluate if those antiseptics provide a long-lasting antibacterial protection effect.
After approval by the research committee, 10 volunteers were recruited for this experimental study. A simple randomization with no repetitions was carried out for 3 antiseptics. The experiment was conducted during a suturing workshop in an experimental laboratory fitted with the regular equipment for surgeries and hand washing stations. The workshop consisted of 3 different suturing sessions (1 hour each), where subjects were subjected to a supervised surgical hand washing protocol before starting their suturing practice. Samples were taken from the finger pulps before (t0) and after (t1) each suture.
The inclusion criteria were: medical students with prior experience in hand washing in a surgical setting. Subjects were not allowed to have history of infections in upper limbs or recent trauma in fingers or hands. All subjects were informed about the risks and benefits of the study and informed consent was requested from them to participate in the study.
Upon signing the informed consent, an informative video was shown to them explaining how to do the hand washing according to the protocols involved. The video was played again at the moment of conducting the experiment.
Before each surgical washing protocol, all participants spent 1-minute washing their hands with neutral soap and a sponge brush in order to eliminate the gross contaminants, and a checklist and a timer were used.
The study participants were randomized to 3 hand antisepsis protocols 1 week apart:
Protocol A: 3 minutes of traditional washing with BD E-Z Scrub 116 (chloroxylenol 3%; Becton Dickinson, Sandy, UT, USA) followed by 1 hour of suturing practice.
Protocol B: 3 minutes of traditional washing with Antibenzil (benzalkonium chloride at 1%; Farmaceuticos Altamirano, Mexico City, Mexico) followed by 1 hour of basic suturing practice.
Protocol C: 1 minute of antisepsis with Avagard (ethyl alcohol 61%, 1% chlorhexidine gluconate; 3M, St Paul, MN, USA) followed by 1 hour of basic suturing practice.
Finger pulps were gently smeared to get the culture on 2 blood agar dishes (one for each hand) for 5 seconds (t0). Right immediately, participants put the gloves on using a sterile technique and conducted their suturing practices (sutures, knots) as part of the workshop activities. One hour later, their gloves were removed with a sterile technique and smears were taken from both hands to culture them on blood agar dishes (t1).
The blood agar dishes had a commercial preparation (DIBICO; DIBICO SA DE CV, Cuatitlan Izcalli, Mexico) sensitive to Streptococcus pyogenes , Streptococcus pneumoniae , Staphylococcus aureus, Escherichia coli.
Five control tests without hand washing were conducted by placing the finger pulps on 2 agar dishes respectively and making sure the culture was done properly.
Each experiment session was one full week apart to prevent residual factors from some of the antiseptics and thus altering the results.
The investigation coordinators (ID, CM) were present at each session (initial hand washing, surgical antisepsis, drying, gloving, suture practicing, and glove removal) to guarantee the appropriate technique and compliance with sterility procedures.
The agar dishes were delivered to the Microbiology Department and were immediately placed in incubators at 35℃ ± 2℃ at a pressure of 5% of CO2. After a 48-hour incubation, the blood agar dishes were taken out of the incubator and the CFUs were counted manually by a technician blind to the protocols, and the dishes were photographed for digital processing (Fig. 1). With an open source software, Open CFU (Quentin Gleissman, Sheffield, UK), the measurements were verified and the interclass correlation coefficient, between the observer and the software, was estimated.
The CFU count distribution was expressed as a mean and as a standard deviation. The before-after comparison for paired data was made with the Wilcoxon signed-rank test. The comparison among groups was performed with the Friedman test and a Bonferroni adjustment was made to delimit the differences among the groups with a P < 0.015, which was considered significant. The entire statistical analysis was conducted by using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA).
Ten subjects were included with a mean age of 21.1 ± 1.3 years, 50% of the participants were women. No adverse events were found in any subject.
The results of the comparison of the antiseptic efficacy in each participant, and in each hand, are shown on Table 1.
At the initial time (t0) with protocol A the CFU count was 82.8 ± 1.3; with protocol B, 9.7 ± 30; with protocol C, 0.1 ± 0.3 (P < 0.001). At the end of the suturing practice session, the CFU count measurements were (t1) for protocol A, 80.7 ± 89.4; protocol B, 7.5 ± 32; protocol C, 0.0 ± 0.0 (P < 0.001).
In order to validate our measurements, the CFU manual measurement was compared to that of the Open CFU software and the interclass correlation coefficient was 0.99.
Expressed as ratios, in protocol A there was bacterial growth in 100% of the agar dishes, in protocol B at the initial time, 40% of subjects had CFU and at the final time, 30%. In protocol C, there was only one CFU in 2 subjects at the initial time and none at the final time.
We did not find any reduction in the amount of CFUs accounting for a statistically significant difference in any anti septic over time (Fig. 2). The most efficacious antiseptic for CFU prevention was the one in protocol C, and there was a sta ti stically significance both at the initial and final periods (P < 0.001).
Hand antisepsis among staff dealing with surgeries is crucial in preventing SSIs. In this experimental study, we compared 3 antiseptics commonly used in our region. We assigned the order of the surgical antisepsis protocols randomly and we measured them before and after a suturing practice session. We found that the combination of alcohol at 61% and chlorhexidine for 1 minute, is more efficacious than other antiseptics available.
Other authors have reported this finding. In a study from Singapore, where povidone iodine was compared against chlorhexidine and alcohol in 10 subjects, the efficacy to prevent CFU growth was higher in the latter group. Interestingly, povidone iodine enhanced the antiseptic protection over time and eliminated the CFU development one hour after the hand preparation [18].
In a study done in Taiwan by Tsai et al. [19], in a clinical trial with 236 patients, povidone iodine was compared to soap with chlorhexidine and ethyl alcohol with chlorhexidine gluconate. In this study they found that the amount of CFUs growth with soap and chlorhexidine and chlorhexidine/alcohol was more efficacious than povidone iodine.
Herruzo-Cabrera et al. [20], compared 4 alcohol solutions to regular washing products (chlorhexidine and povidone iodine) in vitro (pig skin) and in vivo. The in vivo test was performed on healthy individuals with a cross-over design, with 154 members of the surgical team, on whom the microbial flora was measured before and after antisepsis, and before and after surgery. In this study, the quantitative, semiquantitative, and qualitative results favored the solution with alcohol without brushing on volunteers and surgeons alike.
In an interesting study carried out by Pietsch [21], an alcohol-based antiseptic was compared to a water-based one and the degree of skin irritation, efficacy in reducing bacteria before and after surgery, were compared. Also, an alcohol-containing antiseptic was compared to alcohol-containing antimicrobial gels. Fewer adverse events were seen in subjects who used an alcohol-based antispectic with lower rates of bacterial growth compared to the water-based solution and gels.
Hajipour et al. [22] described in a randomized clinical trial the comparison between chlorhexidine gluconate and an alcohol gel as disinfectants. In the first part, all surgeons followed the traditional antisepsis protocol by using chlorhexidine under running water with a brush for 5 minutes in the first case. After the first case, surgeons were divided into 2 groups: one with alcohol gel and another one with chlorhexidine gluconate. At the end of each procedure, a culture was taken from the fingertips of each surgeon. After 41 surgical procedures and 82 hand washings, 4 hands had contamination (8%) compared to 19 hands in the group that used alcohol gel (34%), and the result was statistically significant. Also, the CFU count was higher in the group that used alcohol gel compared to the group that used chlorhexidine.
Regarding the surgical washing time, Kappstein et al. [23] compared a reduced antisepsis time (3 and 2 minutes) to the 5-minute standard time. The protocols they used were: 3 minutes with alcohol disinfectant, and 2 minutes with chlorhexidine and alcohol at 70%. They found that the time-reduced protocols were equally effective as the traditional procedure and that 2,360 hours and 79,768 German marks could be saved or 3,540 hours and 119,652 German marks, respectively.
In this experiment, to eliminate the risk of contamination, we took great care to carefully apply the antiseptic method as described by each of the manufacturers. A research coordinator, was in charge of verifying the proper technique of hand antisepsis that was done after thoroughly washing both hands with a normal soap. After meticulous culturing, the petri boxes were photographed. Two independent observers quantified the colonies that grew, and computer software was explored to leave human error or bias out of the equation. We consider that the result in a single subject in B antiseptic, and all patients with A antiseptic, can reflect potential problems in a surgical setting. Although contamination remains as a possibility, our concern is that antiseptics may have suboptimal results, and/or bacterial resistance to antiseptics is present [242526].
Our study has some limitations. It was performed at the surgical laboratory of our school and its applicability to the clinical setting is difficult. The sample size is small, but given the large differences found among the groups, it is statistically sufficient. Since this was a pilot study, we did not identify the specific bacteria in order to determine the potential species of the infections in our region. Also, due to the lack of patients, we could not measure the extrinsic risk factors involved in the development of SSIs.
With our results, we observed that the alcohol-chlorhexidine combination significantly reduces CFU growth with a continuous elimination of bacteria lasting several hours after being applied. We believe that the relatively short application time could optimize the process and use of hospital resources, which could result in enhanced adherence by hospital staff. Traditional washing, on the contrary, requires vigorous and lengthy rubbing with brushes and antimicrobial solutions, which could cause significant damage to the skin, and is associated to higher costs of supplies such as brushes, sterile towels, running water at sinks.
In conclusion, according to our pilot study, rubbing the hands with an alcohol-based solution with an additional compound (chlorhexidine) showed superior efficacy in the reduction and maintenance of CFUs compared to benzalkonium chloride, and to chloroxylenol brush.
Figures and Tables
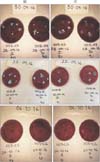 | Fig. 1Colony forming units on blood agar dishes. A, chloroxy lenol; B, benzalkonium chloride; C, alcohol 61% y chlorhexidine at 1%. D, right, I, left. Before the practice (t0) after the practice (t1). |
 | Fig. 2Bar graph comparing the amount of CFUs with 3 antiseptics: A, chloroxylenol; B, benzalkonium chloride; C, alcohol 61% y chlorhexidine at 1%. Note that in groups B and C there was a reduction in CFUs, however almost null growth was observed in group C. Values are presented as mean ± 2 standard deviation. *Wilcoxon signed test A vs. C t1, P < 0.001. †Wilcoxon signed test B vs. C t1, P = 0.027. t0, time zero; t1, time after practice. |
ACKNOWLEDGEMENTS
To the medical students of the “Dr. Alberto Romo Caballero” School: Alejandra Anzures, Rebeca Martinez Leija, Lidia Meraz, Dafne Bizueth, Edilberto Cruz Perez, Dr. Argüelles, Dr. Sierra for their valuable participation and guidance in this work.
We express our gratitude to Laura Martínez Conchos and Universidad Autonoma de Tamaulipas for funding this project.
References
1. Delgado-Rodriguez M, Gomez-Ortega A, Llorca J, Lecuona M, Dierssen T, Sillero-Arenas M, et al. Nosocomial infection, indices of intrinsic infection risk, and inhospital mortality in general surgery. J Hosp Infect. 1999; 41:203–211.
2. Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011; 377:228–241.

3. National Institute for Health and Clinical Excel lence. Surgical site infection: preven tion and treatment of surgical site infec tion Clinical Guideline 74. London: National Institute for Health and Clinical Excellence;2008.
4. Leaper DJ, Tanner J, Kiernan M, Assadian O, Edmiston CE Jr. Surgical site infection: poor compliance with guidelines and care bundles. Int Wound J. 2015; 12:357–362.

5. Plowman R, Graves N, Griffin M. The socio-economic burden of hospital acquired infection. London: Public Health Labo ratory Service;2000.
6. Fry DE. The economic costs of surgical site infection. Surg Infect (Larchmt). 2002; 3:Suppl 1. S37–S43.

7. Gillespie BM, Chaboyer W, Erichsen-Andersson A, Hettiarachchi RM, Kularatna S. Economic case for intra operative interventions to prevent surgical-site infection. Br J Surg. 2017; 104:e55–e64.
8. World Health Organization. WHO guide lines on hand hygiene in health care. WHO Guideline series. Geneva (Switzerland): World Health Organization;2009.
9. Australian College of Operating Room Nurses. Standards for perioperative nursing. O'Halloran Hill (Australia): ACORN Ltd;2012.
10. Conner R, Burlingame B, Denholm B, Link T, Ogg MJ, Spruce L, et al. Perioperative standards and recommended practices. Denver (CO): AORN;2014.
11. Association for Perioperative Practice. Standards and recommendations for safe perioperative practice. Harrogate (UK): Asso ciation for Perioperative Practice;2011.
12. Oriel BS, Chen Q, Itani KM. The impact of surgical hand antisepsis technique on surgical site infection. Am J Surg. 2017; 213:24–29.

13. Tanner J, Dumville JC, Norman G, Fortnam M. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst Rev. 2016; (1):CD004288.

14. Hobson DW, Woller W, Anderson L, Guthery E. Development and evaluation of a new alcohol-based surgical hand scrub formulation with persistent antimicrobial characteristics and brushless application. Am J Infect Control. 1998; 26:507–512.

15. Larson EL, Butz AM, Gullette DL, Laughon BA. Alcohol for surgical scrubbing? Infect Control Hosp Epidemiol. 1990; 11:139–143.

16. Hibbard JS, Mulberry GK, Brady AR. A clinical study comparing the skin antisepsis and safety of ChloraPrep, 70% isopropyl alcohol, and 2% aqueous chlorhexidine. J Infus Nurs. 2002; 25:244–249.

17. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999; 12:147–179.

18. Lai KW, Foo TL, Low W, Naidu G. Surgical hand antisepsis-a pilot study comparing povidone iodine hand scrub and alcohol-based chlorhexidine gluconate hand rub. Ann Acad Med Singapore. 2012; 41:12–16.
19. Tsai JC, Lin YK, Huang YJ, Loh EW, Wen HY, Wang CH, et al. Antiseptic effect of conventional povidone-iodine scrub, chlorhexidine scrub, and waterless hand rub in a surgical room: a randomized controlled trial. Infect Control Hosp Epidemiol. 2017; 38:417–422.

20. Herruzo-Cabrera R, Vizcaino-Alcaide MJ, Fdez-Acinero MJ. Usefulness of an alcohol solution of N-duopropenide for the surgical antisepsis of the hands compared with handwashing with iodine-povidone and chlorhexidine: clinical essay. J Surg Res. 2000; 94:6–12.

21. Pietsch H. Hand antiseptics: rubs versus scrubs, alcoholic solutions versus alcoholic gels. J Hosp Infect. 2001; 48:Suppl A. S33–S36.

22. Hajipour L, Longstaff L, Cleeve V, Brewster N, Bint D, Henman P. Hand washing rituals in trauma theatre: clean or dirty? Ann R Coll Surg Engl. 2006; 88:13–15.

23. Kappstein I, Schulgen G, Waninger J, Daschner F. Microbiological and economic studies of abbreviated procedures for surgical hand disinfection. Chirurg. 1993; 64:400–405.
24. Oriel BS, Chen Q, Wong K. Itani KMF. Effect of hand antisepsis agent selection and population characteristics on surgical site infection pathogens. Surg Infect (Larchmt). 2017; 18:413–418.

25. Noguchi N, Hase M, Kitta M, Sasatsu M, Deguchi K, Kono M. Antiseptic susceptibility and distribution of antiseptic-resistance genes in methicillinresi stant Staphylococcus aureus. FEMS Microbiol Lett. 1999; 172:247–253.
26. Mayer S, Boos M, Beyer A, Fluit AC, Schmitz FJ. Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and -susceptible Euro pean isolates of Staphylococcus aureus. J Antimicrob Chemother. 2001; 47:896–897.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download