INTRODUCTION
METHODS
Study design
Participants
Participants are included if all following criteria are met:
- Agreement to participate in the study and signed the consent form
- A diagnosis of suspicious of malignancy or malignancy (Bethesda category V or VI) based on fine needle aspiration (FNA), or suggestive of malignancy or malignancy by core needle biopsy
- Age of ≥18 years
- A maximum nodule diameter of ≤1 cm
The overall exclusion criteria were:
- Unable or unwilling to attend regular follow-ups
- A diagnosis of benign, atypia of undetermined significance, suspicious for follicular neoplasm, or follicular neoplasm (Bethesda category II, III, or IV) based on FNA or, or benign, indeterminate by core needle biopsy
Patients were not excluded if they had:
- ≥2 unilateral or bilateral PTMCs
- Cytologically or histologically confirmed benign >1 cm nodules with intermediate or highly suspicious ultrasonographic findings (Korean Thyroid Imaging Reporting and Data System [K-TIRADS] Class 4–5)
- Cytologically unconfirmed >1 cm nodules with ultrasonographic findings suggestive of benign lesions (K-TIRADS Class 2–3)
- Hypothyroidism or hyperthyroidism
- Pregnancy before or during the study
- A family history of non-medullary thyroid cancer
Participants were allowed to choose AS if they fulfilled the following criteria:
- No evidence of organ involvement (e.g., esophagus, nerves, trachea, major vessels, or muscle) in imaging studies including high-resolution of ultrasonography (PTMC adjacent or abutting to organs were included if it is not suspected of organ involvement)
- No clinically suspicious or pathological diagnosis of lymph node/distant metastasis
- No poorly differentiated cancer or variant with a poor prognosis, such as the tall cell, diffuse sclerosing, columnar cell, or solid variants
Participants were recommended to undergo immediate surgery if they fulfilled the following criteria:
- Suspected organ involvement (e.g., trachea, esophagus, nerves, vessels, or muscles)
- Clinical suspicious or pathological diagnosis of lymph node/distant metastasis
- Poorly differentiated histology or a variant with a poor prognosis such as tall-cell, diffuse sclerosing, columnar cell, or solid variant
- Presence of Graves' disease with an indication for radioactive iodine therapy or surgery
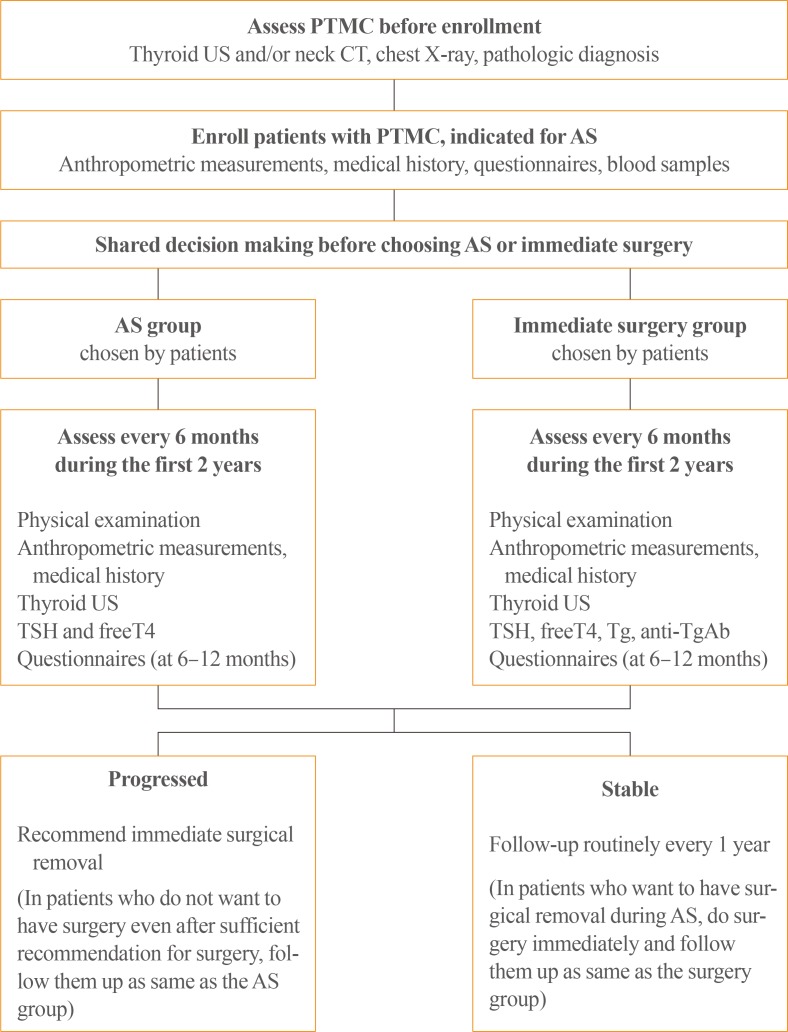
Enrollment and follow-up
Disease progression is defined as:
- A size increase of ≥3 mm in at least one dimension, or ≥2 mm in at least two dimensions
- Suspected organ involvement during the follow-up, such as trachea, esophagus, nerves, vessels, or muscles in imaging study including high-resolution ultrasonography
- Pathological diagnosis of lymph node/distant metastasis
Patients in the AS groups are also recommended to undergo surgery if:
- They elect to undergo surgery in the absence of progression
- Combined Graves' disease is suitable for radioactive iodine ablation therapy or surgery
- Clinical symptoms (hoarseness or dysphagia) during a vocal cord inspection suggest recurrent laryngeal nerve involvement.
Outcomes
Data collection
Clinical data
Laboratory data
Imaging data
Sample size
Data analysis
RESULTS
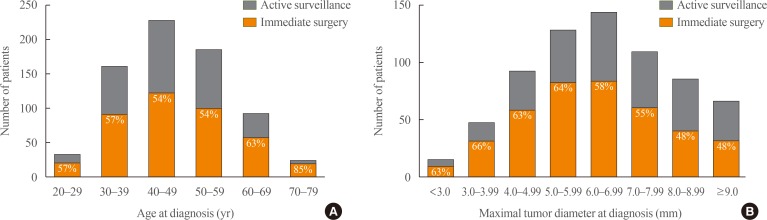 | Fig. 3Distribution of (A) age and (B) tumor diameter at diagnosis according to active surveillance and immediate surgery group. |
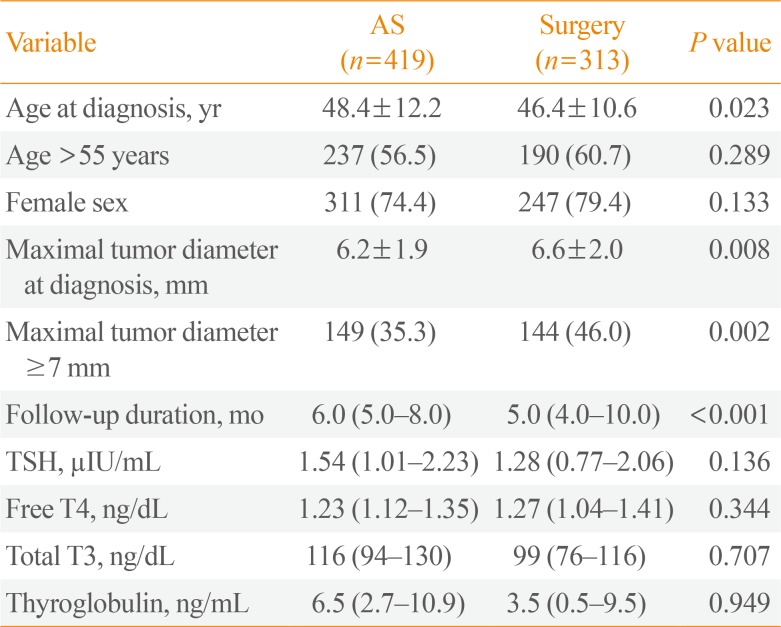
Table 1
Baseline Clinicopathologic Features in Patients with Papillary Thyroid Microcarcinoma According to Active Surveillance and Immediate Surgery Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download