TO THE EDITOR: It has been suggested that short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate, which are produced by the microbial fermentation of dietary fiber in the gut, exhibit several immunologic or metabolic effects. For example, SCFAs can modulate neutrophil migration [
12], induce the differentiation of regulatory T cells [
3], and inhibit tumor necrosis factor-α production from macrophages [
45].
Recently, Vieira et al. [
6] reported that treatment with acetate accelerated the resolution of inflammation in experimental mouse models of gout. The effects of acetate were shown to be mediated by accelerated neutrophil apoptosis, enhanced efferocytosis, reduced nuclear factor-κB activity, and an enhanced production of anti-inflammatory mediators including interleukin-10, transforming growth factor-β, and annexin A1.
Neutrophils exert their anti-microbial functions through the formation of neutrophil extracellular traps (NETs), the production of reactive oxygen species, and the secretion of several proteases such as elastase, lactoferrin, and lysozyme. NETs are composed of decondensed chromatin fibers coated with antimicrobial proteins such as myeloperoxidase, neutrophil elastase, and α-defensin [
7]. However, NETs contribute to organ damage including acute lung injury, thrombosis formation, autoimmune pathologies, metastasis of malignant tumors, and atherosclerosis [
7]. In this study, we investigated the effects of acetate on the formation of NETs.
The following methods were utilized. Heparinized peripheral blood was collected from healthy volunteers after obtaining their written informed consent. Neutrophil separation (>90% purity) was performed as reported previously [
8]. This study was approved by the Ethics Committee of Himeji Dokkyo University (12-01, 17-08).
Phorbol myristate acetate (PMA), bovine serum albumin, nuclease from Staphylococcus aureus, ethylene glycol tetraacetic acid, diphenylene iodonium (DPI), and acetate were purchased from Sigma-Aldrich (St. Louis, MO, USA). SYTOX Green and SYTO 59 Red were obtained from Molecular Probes (Eugene, OR, USA).
Quantitative analysis was performed as described by Palmer et al. [
9] with partial modification as reported previously [
8]. Briefly, a 96-well plate was coated with 1% bovine serum albumin and 0.2 mL of purified neutrophils suspended in Roswell Park Memorial Institute 1640 medium was added to each well. The cells were stimulated with PMA for 3 hours at 37℃ in humidified air with 5% CO
2 and acetate was simultaneously added. After nuclease treatment, the cells were transferred to Eppendorf tubes and centrifuged at 1,800× g for 10 min at 4℃. SYTOX Green, which is a nucleic acid stain that cannot permeate live cells, was added to the supernatants at a final concentration of 2.9 µM and fluorescence was detected at excitation and emission wavelengths of 488 and 520 nm, respectively. Relative fluorescence was then calculated by comparison with controls. The linearity of this method was confirmed again [
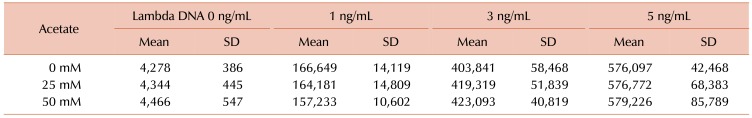
8] and the addition of 25 or 50 mM acetate to this system did not alter the results (
Table 1).
Table 1
Lambda DNA was measured using SYTOX Green.

For morphological analysis, purified neutrophils were suspended in Hank's balanced salt solution with 5% heat-inactivated autologous serum at 1×10
9 cells/L. This cell suspension (250 µL) was then added to 35-mm glass-bottomed dishes coated with poly-ʟ-lysine (Sigma-Aldrich) and incubated under the same conditions as those used for quantitative analysis. Next, SYTOX Green and SYTO 59 Red, which are both cell-permeable nucleic acid stains, were added at a concentration of 4 µM and stained neutrophils were observed under a fluorescence microscope (TH4-100 Cell Sens; Olympus, Tokyo, Japan) [
8].
Superoxide production was detected by means of chemiluminescence with a device manufactured by Hamamatsu Photonics (Hamamatsu, Japan) as previously reported [
10]. EZR (Easy R) was used for all statistical analyses [
11], and paired t-tests were used to compare data from the two groups.
The following results were obtained. At concentrations exceeding 0.5 mM, acetate significantly inhibited PMA-induced NETs generation (
Fig. 1, N=3–4) in a dose-dependent manner. However, the inhibition exerted by acetate was less potent than that exerted by DPI, which is a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor. A trypan blue dye exclusion test revealed that more than 98.5% of the cells were viable after 3 hours of incubation with 25 mM acetate. Fluorescence microscopic observation also revealed the inhibitory effect of acetate on PMA-induced NETs (
Fig. 2). Micrographs are shown of the control (
Fig. 2A), acetate treatment (25 mM) alone (
Fig. 2B), the positive control treated with 50 nM PMA (
Fig. 2C), and the combination of PMA and acetate (
Fig. 2D).
Figs. 2E-H show the corresponding SYTO 59 Red images of the corresponding areas and
Figs. 2I-L show phase-contrast micrographs. These findings clearly indicate that treatment with 25 mM acetate attenuated PMA-induced NETs formation.
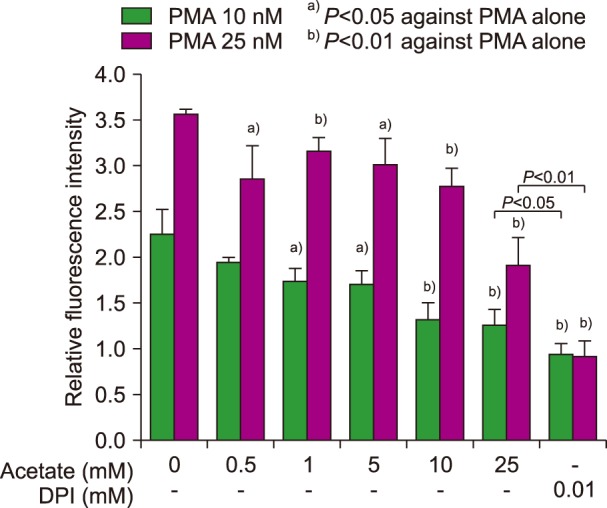 | Fig. 1Effects of short-chain fatty acids on NET generation and reactive oxygen species production. A quantitative analysis revealed that acetate had a dose-dependent inhibitory activity on NETs generation, although its potency was lower than that of DPI.
|
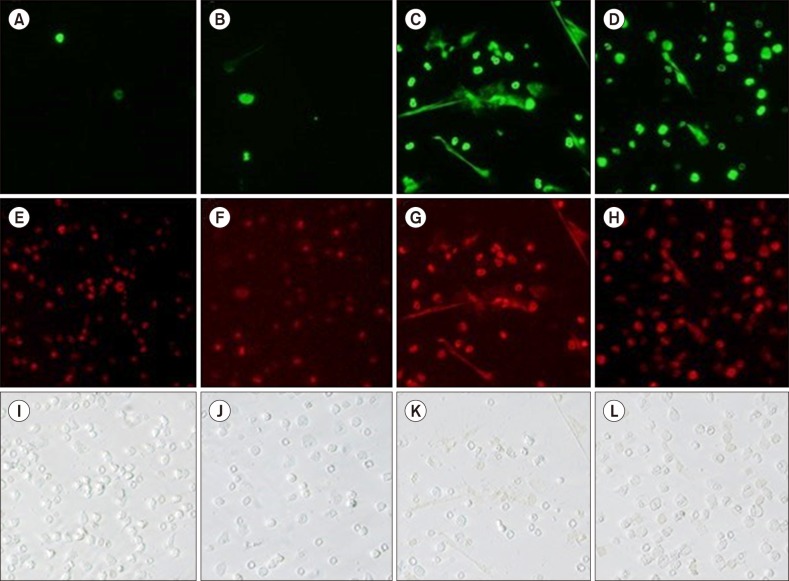 | Fig. 2Morphological observation by fluorescence microscopy. SYTOX Green staining revealed the ability of acetate to inhibit NETs. (A, E, I) Control; (B, F, J) 25 mM acetate alone; (C, G, K) 50 nM PMA alone; (D, H, L) 50 nM PMA with 25 mM acetate. (A-D, SYTOX Green staining) (E-H, SYTO 59 Red staining) (I-L, phase-contrast micrographs, ×100).
|
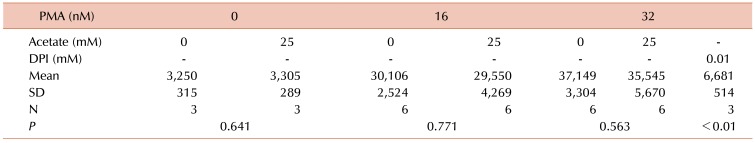
Since reactive oxygen species production is a prerequisite for PMA-induced NETs formation, the effects of acetate on superoxide formation were assessed by chemiluminescence. As shown in
Table 2, acetate did not inhibit the induction of superoxide formation by either 8 or 16 nM PMA.
Table 2
Superoxide was detected using chemiluminescence.

The concentration of acetate in the portal vein is reportedly 0.1–0.3 mM and that in the lumen of the colon is 20–140 mM [
12]. In our experiments, low concentrations of acetate weakly inhibited PMA-induced NET formation, while higher concentrations similar to those available in the colonic lumen moderately inhibited NET formation, although the inhibitory effect of acetate was weaker than that of DPI. Considering these results, the biological significance of SCFAs in inflammatory bowel diseases (IBDs) is of interest. In the pathogenesis of IBDs such as ulcerative colitis and Crohn's disease, neutrophil activation is thought to result in tissue damage. NET production, in particular, has been shown to be involved in the thromboembolic events that are frequently observed in IBD patients [
13]. Although pharmaceutical approaches for the control of IBDs have improved, a high proportion of patients remain refractory to current drugs. However, ongoing human studies have indicated that interventions with dietary fiber, which induces SCFAs generation through fermentation, show beneficial outcomes for IBDs [
14]. The moderate inhibition of NETs generation by acetate as described here may be relevant to these clinical observations. Thus, studies are needed to determine the effects of combined treatments with acetate and other neutrophil-inhibitory substances such as antioxidants [
15].
The effects of acetate are reportedly related to the G-protein-coupled receptor GPR43, which has been shown to be expressed on neutrophils [
2]. Based on these findings, it is necessary to clarify the mechanism by which SCFAs can inhibit the activation of neutrophils.
Conclusion
NET formation is moderately inhibited by acetate through a currently unknown mechanism that is unrelated to NADPH oxidase inhibition.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download