TO THE EDITOR: Microangiopathic hemolytic anemia (MAHA) refers to mechanical hemolytic anemia characterized by red blood cell fragmentation or schistocytes on peripheral blood (PB) smear [1]. MAHA is observed in various conditions such as thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome (HUS), disseminated intravascular coagulation (DIC), systemic infection, and immune disorders [234]. MAHA can also occur rarely in malignant tumors as paraneoplastic syndrome. Gastric cancer is the most frequent malignancy associated with MAHA, followed by cancers of the breast, prostate, lung and cancer of unknown origin. Colon cancer presenting with MAHA is much rarer, and only a few cases have been reported [235]. In most cancer-related MAHA (CR-MAHA) cases, MAHA is detected at initial diagnosis of cancer. However, in about 20% of cases, it emerges at the time of cancer recurrence, particularly in gastric and breast cancers, and usually reflects late stage of disease [5]. When MAHA presents as the first sign of recurrent malignancy, the underlying cause may not be suspected initially, leading to inappropriate management. Herein, we describe a case of relapsed colon cancer which presented no sign of recurrence except MAHA as the initial manifestation.
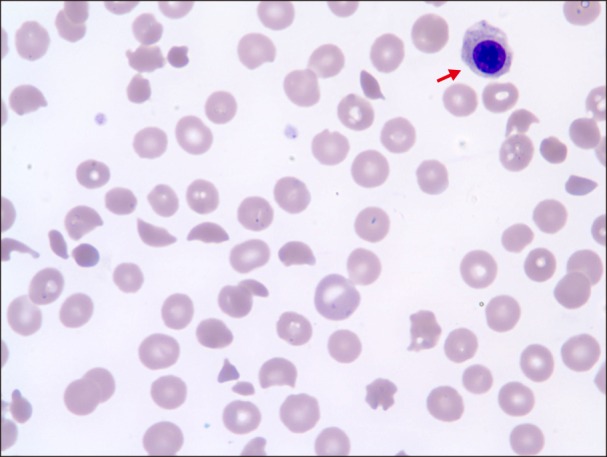
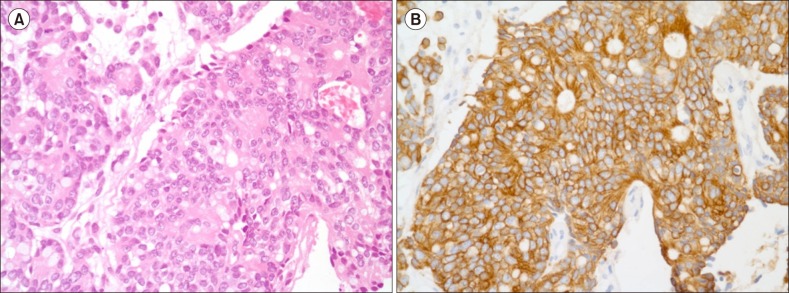
A 76-year-old woman was admitted to our hospital with ecchymosis that developed suddenly all over her body accompanied by headache. Three years ago, she was diagnosed with colon cancer and underwent hemicolectomy followed by adjuvant chemotherapy with leucovorin and 5-fluorouracil. Since then, the patient was regularly followed up. The last checkup was 7 weeks ago, and no evidence of disease recurrence was seen on abdominopelvic computed tomography (CT) scan. Blood cell counts at that time were: white blood cells (WBC), 4.88×109/L; hemoglobin (Hb), 11.4 g/dL; and platelets, 117×109/L. Chemistry results were unremarkable. Her complete blood count (CBC) on admission was as follows: WBC, 5.9×109/L; Hb, 6.7 g/dL; and platelets, 4×109/L. Other significant laboratory results included lactate dehydrogenase (LDH), 2,016 U/L [normal level (NL), 240–480 U/L]; alkaline phosphatase (ALP), 262 IU/L (NL, 35–104 IU/L); total bilirubin, 2.86 mg/dL (NL, 0.2–1.2 mg/dL) with direct bilirubin, 0.35 mg/dL (NL, 0–0.3 mg/dL); prothrombin time (PT), 18.2 s (NL, 9–13 s); activated partial thromboplastin time (aPTT), 39.0 s (NL, 25–37 s); D-dimer, 36,641 ng/mL (NL, 0–250 ng/mL); haptoglobin, 4 mg/dL (NL, 30–200 mg/dL) and a negative direct Coombs' test. PB smear showed numerous fragmented erythrocytes, polychromasia, nucleated red blood cells (RBCs) (Fig. 1), and leukoerythroblastic reaction. A diagnosis of MAHA was made on the basis of laboratory findings. Brain CT scan revealed acute subdural hemorrhage (SDH) on both fronto-parieto-temporal lobes. Abdominopelvic CT scan and whole spine X-ray showed no evidence of newly appeared distant metastasis of cancer. Urgent neurosurgery was scheduled; however, it was withheld due to low platelet count. Despite multiple blood transfusions, her blood counts did not improve. Schistocytosis and leukoerythroblastic reaction were still observed on a repeat PB smear, and values of other significant laboratory parameters such as LDH and total bilirubin continued to worsen, implying a progression of MAHA. Then, bone marrow (BM) examination was performed for the workup of MAHA and thrombocytopenia; however, BM aspirate showed no adequate particles with dry tap. While awaiting the slide preparation of the biopsy section, plasma exchange was planned with a diagnostic consideration of TTP. Unexpectedly, the biopsy section revealed diffuse infiltration of atypical mononuclear cells, suggestive of BM involvement of malignant tumor (Fig. 2A). Hence, plasma exchange was withheld. With rapidly progressive clinical course, the patient expired on the 10th day since admission. The results of immunohistochemical staining arrived a few days later, and the atypical cells were positive for CK20 stain (Fig. 2B) and negative for CK7 stain. A final diagnosis of BM metastasis of poorly differentiated adenocarcinoma clinically from colon was made.
MAHA can be seen in various diseases which share similar laboratory and clinical features. In patients with an abrupt onset of MAHA and thrombocytopenia, a diagnosis of TTP and urgent management such as plasma exchange should be preferentially considered. Because TTP is a medical emergency, complete presentation of the classic pentad of TTP including MAHA, thrombocytopenia, fever, neurological symptoms and renal disease may not be strictly required for its clinical diagnosis. CR-MAHA with no apparent systemic manifestation of malignancy may be often included in such cases. This leads to the trial of plasma exchange, which is seldom effective and may cause unnecessary risk of complication in CR-MAHA cases [678]. Presenting laboratory findings in this case are indicative of overt DIC, although a diagnosis of DIC should be based on both clinical and laboratory features. A close association of CR-MAHA with DIC is well described [356]. According to a multicenter study involving 12 cases of CR-MAHA, all cases showed severely increased D-dimer levels [2]. It was suggested that the generation of small vessel thrombi in CRMAHA may be increased by simultaneous DIC. Extreme elevation of D-dimer, as in this case, may support disseminated malignancy rather than TTP, even though no single test can establish or exclude the diagnosis [257].
Although BM metastasis is commonly found in CRMAHA, it is seldom an initial presenting feature of malignant tumor. BM metastasis from colon cancer is very rare, particularly when encountered in the absence of usual distant metastases such as liver and lungs [8910]. Generally, BM study is not routinely considered in solid tumor workup; however, PB slide examination is performed in almost all known or suspected cancer cases as a simple screening tool. One of the well-known PB findings suggestive of BM involvement of malignancy is leukoerythroblastic reaction. Although leukoerythroblastic reaction is not specific for malignant conditions, its presence accompanied by MAHA could be a strong indicator of BM examination in cases with unexplained cytopenia [10].
Chemotherapy is the only effective treatment for CRMAHA. Although the prognosis of CR-MAHA is generally poor, several studies reported cases of favorable response of CR-MAHA to chemotherapy [245]. As MAHA is not a common presenting sign of cancer recurrence, early recognition of this rare presentation and prompt investigation including BM study are essential for timely management.
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Brain MC, Dacie JV, Hourihane DO. Microangiopathic haemolytic anaemia: the possible role of vascular lesions in pathogenesis. Br J Haematol. 1962; 8:358–374. PMID: 14014893.

2. Oberic L, Buffet M, Schwarzinger M, et al. Cancer awareness in atypical thrombotic microangiopathies. Oncologist. 2009; 14:769–779. PMID: 19684072.

3. Alonso JV, Fonseca J, Lopera EL, Aguayo MÁ, Montes YH, Llamas JC. A report of disseminated adenocarcinoma presenting as thrombotic thrombocytopenic purpura. Hematol Rep. 2011; 3:e14. PMID: 22184535.
4. Tang M, Goldstein D. The role of chemotherapy in gastric cancer-related microangiopathic haemolytic anaemia. J Gastrointest Oncol. 2017; 8:E10–E15. PMID: 28280630.

5. Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore). 2012; 91:195–205. PMID: 22732949.
6. Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017; 1:590–600. PMID: 29296701.

7. Lansigan F, Isufi I, Tagoe CE. Microangiopathic haemolytic anaemia resembling thrombotic thrombocytopenic purpura in systemic lupus erythematosus: the role of ADAMTS13. Rheumatology (Oxford). 2011; 50:824–829. PMID: 21149242.

8. Yesodharan J, Kuruvilla S, Parameswaran Kavitha K, Lilly M. Disseminated gastric carcinoma in disguise-presentation as microangiopathic haemolytic anemia with bone marrow necrosis. Transl Gastroenterol Hepatol. 2016; 1:6. PMID: 28138574.

9. Assi R, Mukherji D, Haydar A, Saroufim M, Temraz S, Shamseddine A. Metastatic colorectal cancer presenting with bone marrow metastasis: a case series and review of literature. J Gastrointest Oncol. 2016; 7:284–297. PMID: 27034798.
10. Ozkalemkas F, Ali R, Ozkocaman V, et al. The bone marrow aspirate and biopsy in the diagnosis of unsuspected nonhematologic malignancy: a clinical study of 19 cases. BMC Cancer. 2005; 5:144. PMID: 16262899.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download