INTRODUCTION
Thrombotic microangiopathy (TMA) refers to the constellation of clinical findings with microangiopathic hemolytic anemia (MAHA), severe thrombocytopenia, and organ failure of variable severity. This includes thrombotic thrombocytopenic purpura (TTP), atypical hemolytic uremic syndrome (aHUS), hemolytic uremic syndrome (HUS), and complications of other systemic illnesses or certain medications. In the past, TTP was diagnosed based on the classic pentad (thrombocytopenia, MAHA, neurological features, fever, and renal involvement) [
1]. HUS is defined by the presence of intravascular hemolysis, thrombocytopenia and acute kidney failure [
2], and is treated presumptively according to presence or absence of bloody diarrhea [
1]. The clinical relevance of “diarrhea-negative” HUS is unclear. Currently, the diagnostic criteria have been changed to thrombocytopenia and MAHA with clinical or histological complications of microvascular thrombosis [
1]. The evaluation of ADAMTS-13 (a disintegrin-like metalloprotease with a thrombospondin type 1 motif 13) activity levels confirming the diagnosis of TTP has revolutionized the definition, which renders the classic pentad seen only in 5% of patients [
3] irrelevant to current clinical practice. The presence of severe ADAMTS-13 activity deficiency (ADAMTS-13<10%) which is the sole biologic marker specific for TTP has become instrumental recently in completing the definition of TTP [
4]. Patients who clinically masquerade as TTP with non-severe ADAMTS-13 activity deficiency (ADAMTS-13≥10%) will be classified as TMA.
There is an old belief that TTP, if left untreated, is associated with 90% mortality rate. However, the advent of ADAMTS-13 activity results in the further classification of TMA into ADAMTS-13 activity deficient (<10%) or non-deficient (≥10%) or slightly deficient categories. Plasma exchange (PEX) therapy is always the first empirical treatment for acute TTP which dramatically improves the prognosis, allowing a better survival of 85% [
45]. However, recent evidence shows that patients with TMA arising from systemic illness usually have normal or slightly deficient ADAMTS-13 activity and respond poorly to PEX therapy. This led to the new belief that treatment should be directed to the underlying disease or stopping the offending drug [
5].
In Malaysia, no study has addressed the epidemiology of TMA yet. This study aimed to research the demographic data, outcomes, as well as the etiology of TMA in the Malaysian population.
Go to :

MATERIALS AND METHODS
Ampang Hospital is the only national referral center for ADAMTS-13 activity testing in Malaysia. This retrospective cohort study involved 243 patients diagnosed with TMA, from 2012 to 2016. All the ADAMTS-13 activity and inhibitor results were traced from the hemostasis laboratory archived records at Ampang Hospital. The medical records were then retrieved through the electronic hospital information system (EHIS) of Ampang Hospital and other hospital records units nationwide. All the demographic data, details of clinical presentation, laboratory results, and treatment were recorded in an Excel spreadsheet before analysis. The flow of the method is displayed in
Fig. 1.
 | Fig. 1Flow chart for the methods.
|
The Technozym method was used to evaluate ADAMTS-13 activity and ELISA (Technoclone GmbH, Wien, Austria) was used to evaluate the inhibitor level. Subsequently, the TMA cases were further divided into different types according to the clinical history, laboratory investigation and ADAMTS-13 results.
Primary acquired TTP is defined as TMA with ADAMTS-13 activity <10% with the presence of inhibitor. Congenital TTP is defined as TMA with ADAMTS-13 activity <10% with absence of inhibitor. The normal range for ADAMTS-13 activity is 40% to 130%. The ADAMTS-13 inhibitor is negative at less than 12 units/mL, borderline positive at 12 to 15 units/mL, and positive at more than 15 units/mL.
Each individual in Malaysia has a unique national identity number, which can be utilized for cross-checking with the National Death Registry from the National Registration Department, Malaysia for survival outcomes (death or alive). By cross-checking with the death registry, information on the date of death was obtained up to 31 December 2016 (end of follow-up). The overall survival (OS) was measured from the date of diagnosis until death from any cause, with observations censored for patients at the date they were last known to be alive. The OS of patients was estimated using the Kaplan-Meier (KM) product limit estimator, and differences in OS between groups were tested using the log-rank test at a significance level of 0.05. The survival data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Besides, the difference in mortality was also tested using Pearson's chi-squared test. Odds ratios (ORs) were estimated for patients with ADAMTS-13 activity <10% versus ≥10% and <5% versus ≥5%.
The study was registered under the National Medical Research Register (NMRR) in Malaysia, and was approved by the Medical Research & Ethics Committee (MREC), a centralized independent ethics committee for public institutions in the country. The Investigator Initiated Research number was NMRR-16-2824-31410. The requirement of informed consent was waived as the study only required information from patients' medical records, laboratory records and the National Death Registry.
Go to :

RESULTS
The overall characteristics of patients with TMA are presented in
Table 1. There was a total of 243 patients undergoing ADAMTS-13 activity testing with the median age of 34.2 years. Females (57.6%) were more predominant in comparison to males (42.4%). In the age groups, young adults (25–44 yr) were the most prevalent group at 34.1%, followed by 27.2% for middle-aged adults (45–64 yr), 19.7% for adolescents (15–24 yr), 12.8% for children (0–14 yr), and 6.2% for elderly adults (≥65 yr). Besides, we also stratified the patients according to the three main ethnic groups in Malaysia. There were 62.5% of Malay, 23.5% of Chinese, 8.6% of Indian and 5.4% of the others, which consisted of natives from the Eastern Peninsular.
Table 1
The overall characteristics of patients with TMA in Malaysia.
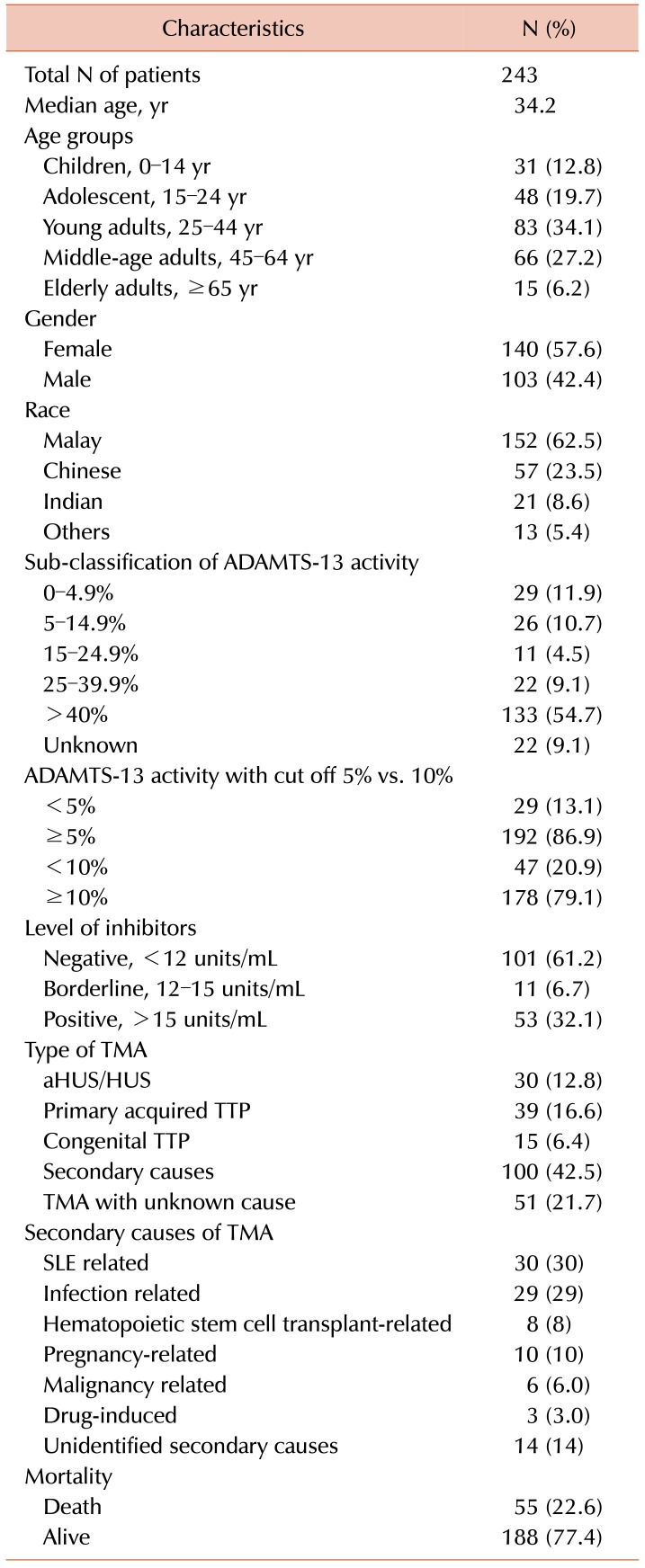

For ADAMTS-13 activity, the results were only available for 225 patients, with 47 cases (20.9%) having ADAMTS-13 activity <10% and 178 cases (79.1%) having ADAMTS-13 activity ≥10%. The ADAMTS-13 testing was started in 2012; there were 18 patients reported with inhibitor level only with the other type of ELISA assay. Some of them were treated as TTP with presence of high inhibitor level. For the consistency of the results, these 18 patients were excluded from subsequent analysis resulting in only 225 patients. Among these 225 patients, 4 patients were reported to have ADAMTS-13 activity <10% so could not be classified into ADAMTS-13 activity <5%, so the number in those with the ADAMTS-13 activity cut off of 5% was only 221. For the validity of the data, we sub-classified ADAMTS-13 activity into different ranges with reference to the
Table 1, with the 18 patients with only inhibitor level reported initially and 4 patients reported with ADAMTS-13 activity <10% (no actual value documented) resulting in 22 patients being categorized as unknown. For ADAMTS-13 inhibitors, the results were only available in 165 cases, with 53 cases (32.1%) being positive, 11 cases (6.7%) being borderline positive and 101 cases (61.2%) being negative for inhibitor levels. Among the TTP cases, 72.2% was acquired, while 27.8% was congenital. There were 10 TTP cases diagnosed based on the detection of high titer of ADAMTS-13 inhibitor level without testing the ADAMTS-13 activity; thus, only 47 cases had confirmed ADAMTS-13 activity level <10%.
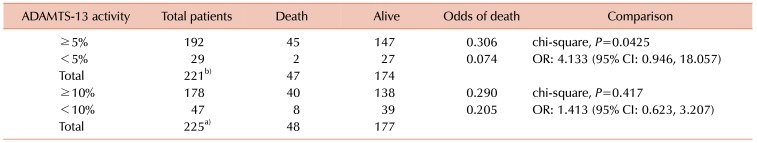
Table 2 summarizes the mortality differences according to different ADAMTS-13 cut-off values. There were 55 (22.6%) mortality cases. Patients with ADAMTS-13 activity ≥5% were associated with higher mortality rate and the odds of death was 4 times higher compared to those with ADAMTS-13 activity <5% [OR: 4.133,
P=0.0425; confidence interval (CI): 0.946, 18.057]. According to the KM survival curve (
Fig. 2A), the OS of those with ADAMTS-13 activity ≥5% was not statistically different from those with ADAMTS-13 activity <5% (log-rank,
P=0.0909). For ADAMTS-13 activity with cut-off of 10%, no significant differences were observed for both mortality (OR: 1.413,
P=0.417) and OS in KM survival curves (log-rank,
P=0.5777) (
Fig. 2B).
 | Fig. 2Overall survival according to the types of TMA. Overall survival according to ADAMTS-13 activity for (A) cut-off=5% and (B) cut-off=10%.
|
Table 2
Results for the difference in mortality using the chi-square test.

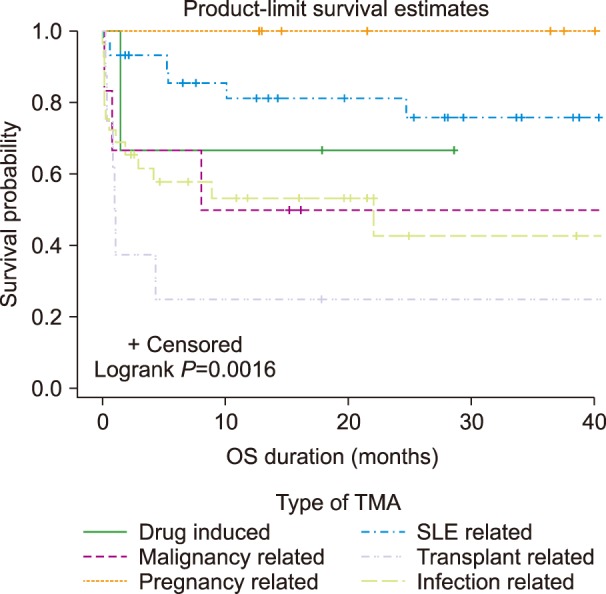
The type of TMA was classified according to the etiologies based on the clinical history, basic blood investigations like full blood count, renal profile, liver function test and ADAMTS-13 activity results, so only a total of 235 patients fulfilled the criteria for the classification. Among the 18 patients that were tested for inhibitor level only, 10 actually had very high inhibitor levels suggested from the clinical history and so were classified as having primary TTP. The other 8 who tested negative for inhibitors and were referred from private hospitals without histories or laboratory investigations were excluded. The most common type of TMA was secondary TMA (42.5%), followed by primary acquired TTP (16.6%), aHUS/HUS (12.8%), and congenital TTP (6.4%). Among the secondary causes, systemic lupus erythematosus presented the highest proportion of 30%, followed by infection (29%), pregnancy (10%), transplant (8%), malignancy (6%), drug-induced (3%), and unidentified secondary causes (14%). Secondary TMA was associated with inferior OS (log-rank,
P=0.0387) (
Fig. 3). Among the secondary causes, transplant-associated TMA (TA-TMA) had the worst OS (log-rank,
P=0.0016), followed by infection-associated and malignancy-associated TMA (
Fig. 4).
 | Fig. 3Overall survival according to secondary causes of TMA.
|
 | Fig. 4Overall survival according to secondary causes of TMA. Overall survival according to ADAMTS-13 activity for (A) cut-off=5% and (B) cut-off=10%.
|
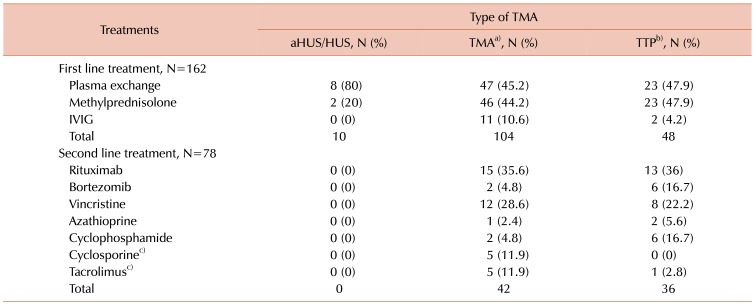
Table 3 summarizes the treatments of TMA. A total of 162 patients were recorded as having received first-line treatments comprising PEX, methylprednisolone and intravenous immunoglobulin (IVIG). PEX and methylprednisolone were given to most of the TMA patients (45.2% and 44.2%, respectively). A total of 48 TTP patients received equal amounts of PEX and methylprednisolone (47.9% each). Only 10 aHUS/HUS patients received PEX and methylprednisolone. IVIG was prescribed to 13 patients only because they were initially treated for idiopathic thrombocytopenic purpura. Further, 10.6% of TMA patients and 4.2% of TTP patients were treated with IVIG. For patients who did not respond to the first-line treatment, the second-line treatment would comprise other immunosuppressants such as rituximab, vincristine, cyclophosphamide, bortezomib and azathioprine, which accounted for a total of 78 patients. Rituximab was used in 35.6% of TMA patients and 36% of TTP patients. In all, 4.8% of TMA and 16.7% of TTP patients were treated with bortezomib. Vincristine was used in 28.6% of TMA patients and 22.2% of TTP patients. Cyclophosphamide was given to 4.8% of TMA patients and 16.7% of TTP patients. Azathioprine was only noted in one TMA patient and two TTP patients. Cyclosporine and tacrolimus were only described in patients with TA-TMA. The first-line treatment for TA-TMA was initially PEX, which was then changed to methylprednisolone-based therapy in view of the ineffectiveness of PEX in this cohort of patients. Cyclosporine was used in 5 patients and tacrolimus was used in 6 patients. Only one patient with TA-TMA underwent PEX as evidenced by the one TTP patient who was treated with tacrolimus. Most of the patients treated with cyclosporine and tacrolimus were TMA patients. Some of these TA-TMA patients received cyclosporine as prophylaxis against graft versus host disease (GVHD), so when they developed TMA, they were switched to tacrolimus based on suspicion of cyclosporine-induced TTP for GVHD treatment. Some of them were continued on cyclosporine, or both cyclosporine and tacrolimus were completely stopped at the discretion of the treating physician.
Table 3
Treatment of TMA patients in Malaysia.

Go to :

DISCUSSION
The etiology of TMA is always complex and challenging to medical practitioners. It is currently viewed as a result of either hemostasis failure (inappropriate platelet activation or thrombin generation) or endothelial injury (exposure to Shiga toxin, uncontrolled complement deposition) [
6]. TTP is defined by the presence of a severe deficiency of ADAMTS-13 activity <10% [
4]. The difference between TTP and aHUS and TMA of others are crucial because therapeutic PEX is only effective in TTP whereas eculizumab has been shown to have superior renal survival as compared to PEX alone [
67]. TMA of other diseases is defined as those without severe ADAMTS-13 deficiency and often requires treatments other than PEX [
48], which is consistent with our study findings.
This study demonstrates that the OS tended to be worse in those with other causes of TMA (ADAMTS-13 activity≥5%) in comparison to those with TTP (ADAMTS-13 activity <5%). This observation was similar in the sub-analysis of the dataset comprising those with complete treatment. However, this is contrary to the United Kingdom prospective TTP registry study in which the mortality was found to be highest among those with ADAMTS-13 antibodies in the highest quartile and antigens in the lowest quartile [
910]. Most of the patients with TMA were treated with PEX in the study (
Table 3). The Harvard TMA Research Collaborative reveals that patients with severe ADAMTS-13 deficiency respond well to PEX, whereas TMA without severe ADAMTS-13 deficiency is associated with increased mortality which may not be influenced by PEX [
11]. Bendapudi et al. (2014) also showed that patients with high PLASMIC scores indicating TTP experienced a far better survival rate than low-scoring patients, as a result of the effectiveness of PEX in treating TTP [
12]. Low-scoring patients had TMA of other causes.
The differential diagnosis of patients presented with MAHA and thrombocytopenia in the current clinical practice is either TTP or HUS/aHUS. In fact, there are many etiologies of TMA, such as TMA in the critically-ill secondary to sepsis, disseminated intravascular coagulopathy, malignant hypertension, pregnancy, drug-induced, cancer-associated, hematopoietic stem cell transplant, cobalamin C defect, and diacylglycerol kinase epsilon deficiency [
5].
In our cohort study, it seems that those with non-deficient ADAMTS-13 activity (ADAMTS-13 activity≥5%) were doing worse than those with typical TTP. Although the statistical analysis yielded insignificant results, it does not mean that the observation was not clinically significant. This is further observed in the analysis of types of TMA whereby the secondary causes of TMA were associated with the lowest OS. Among the secondary causes, TA-TMA had the worst survival outcome. TA-TMA is unique as it has multiple triggers leading to multisystem injury with immune dysregulation caused by infections, chemotherapy, and GVHD, which subsequently results in endothelial injury and complement activation [
5]. Jodele et al. (2015) revealed that the odds of death among subjects with TA-TMA were 5 times higher in the first-year post transplant compared to those without TA-TMA [
13]. Many of our transplant patients were on immunosuppressant therapy for GVHD prophylaxis like cyclosporine and tacrolimus, which were stopped whenever TMA was suspected. However, the discontinuation of the drugs did not seem to improve the outcome, which is also supported by the study by Jodele et al. (2015). In view of the lack of conclusive data, adjunctive therapies like GVHD prophylaxis and treatment should be evaluated carefully in transplant patients individually [
13]. All the 8 cases of TA-TMA were not associated with severe deficiency of ADAMTS-13 activity except one of them who received PEX, so expectedly no improvement in the conditions as illustrated by Matevosyan et al. (2015) that PEX is not indicated [
14]. Some authors advocate that eculizumab has been a promising treatment for TA-TMA [
1314].
Infection-related TMA presented the second highest mortality rate among the secondary causes of TMA. It has been postulated that neutrophils are pivotal in inflammation and thrombosis via the formation of neutrophils extracellular traps (NETs) [
14]. There is evidence showing increased plasma levels of circulating DNA-histone complexes in TMA [
15], which are released from inflammatory cells like neutrophils in response to infection in the form of nucleosomes (NETs). NETs can cause thrombosis by stimulating platelet aggregation and thrombin generation as it contains DNA, histones and neutrophil proteases [
15]. This cohort also includes those with sepsis and critically-ill patients who also do not show a response to PEX resulting in a poor outcome.
aHUS/HUS were diagnosed retrospectively from the nephrology department in which these TMA patients became renal replacement therapy dependent after years of presentation. The lack of Shiga toxin testing secondary to Escherichia coli and complement activation markers not only results in failure to confirm the diagnosis but also in differentiating one from another. Shiga toxin-producing
E. coli (STEC)-HUS occurs as a result of clinical gastroenteritis secondary to STEC (usually serotype 0157:H7 or 0104:H4) which is pivotal, causing endothelial cell damage [
2]. On the other hand, aHUS occurs without any relation to infection or coexisting disease [
16]. There is dysregulation of complement activation either due to genetic or acquired factor in the host cells [
2]. Furthermore, there is also secondary HUS which is triggered by coexisting disease or conditions such as infection with
Streptococcus pneumoniae, influenza virus, transplantation of both solid organs and bone marrow, autoimmune disease, malignancy, pregnancy, and use of some cytotoxic drugs [
2]. It is crucial to reach a diagnosis of aHUS because of the recent discovery of eculizumab for the superior renal outcome of aHUS. The C5 blocking eculizumab therapy has not been shown to be effective in treating STEC-HUS and TTP due to the good response to PEX [
15]. Unfortunately, this drug is not widely accessible in most countries including Malaysia due to the astronomical cost of the drug.
First-line treatments like PEX and steroids were provided to most of the patients with complete treatment dataset. This was only effective in primary TTP but not in our cohort as most of the patients actually had TMA of other causes. The second-line treatment, as illustrated in
Table 3, is only used when there is suboptimal therapeutic response to PEX and steroid therapy as per the institutional protocol. The humanized anti-CD20 monoclonal antibody rituximab is suggested to as a frontline therapy together with PEX as the UK group demonstrated that the upfront treatment with rituximab is associated with shorter hospitalization and fewer relapses in comparison to the standard TTP group without rituximab [
1718]. The is further supported by the French TMA reference center network and the Oklahoma TTP registry showing fewer and later relapses in patients treated with rituximab [
1920]. However, there are no data so far demonstrating the effectiveness of rituximab in TMA with non-deficient ADAMTS-13 activity. Bortezomib is used at our center in the treatment of refractory TTP in which it seems to be effective in the treatment of a subgroup of cases with severe, refractory TTP [
21]. Vincristine was used in the past pre-rituximab era during which stable remission was achieved in 73% of patients receiving vincristine [
17]. The prescription of antiplatelets like aspirin was not widespread in our study because of the concern of thrombocytopenia. Low-molecular-weight heparin was used only in those with venous thromboembolism. In fact, Lapponi et al. (2013) stated that treatment with acetylsalicylic acid (ASA) prevented NET formation [
22]. The anti-inflammatory effects of ASA exert inhibition on NET by acting on cyclo-oxygenase independent targets including the NF-κB [
22].
There are a number of limitations to this study which are related to the retrospective data analysis, single-center design, and inability to calculate population-based rates. The treatment data were very limited and available mostly for patients fully treated at Ampang Hospital. The data from other hospitals had been lacking in treatment details despite repeated tracing. Consequently, there was a variable total number of patients receiving individual treatments depending on the availability of the data resulting in only description. Some of the patients had short durations of follow-up. This study could not determine a cause and effect relationship. However, the capture of the data at specific time points provides us many findings which can be utilized to carry out in-depth research for secondary causes of TMA in future which are not always highlighted in current practice.
TMA could masquerade many diseases beyond what was known about only TTP in the past. This study illustrates that although there is no significant difference in the overall survival, there is a tendency of inferior outcome among those with non-deficient ADAMTS-13 activity in comparison to those with severely deficient ADAMTS-13 activity. Routine standards of care like PEX and immunosuppressant therapy are only effective in genuine TTP. Other types of TMA such as aHUS/HUS, secondary causes of TMA need to be addressed according to the underlying etiology in order to reduce the morbidity and mortality. This clinical observation highlights the need to investigate other types of TMA prospectively in the future.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download