This article has been corrected. See "Erratum: Tolerogenic Dendritic Cells Reduce Airway Inflammation in a
Model of Dust Mite Triggered Allergic Inflammation" in Volume 10 on page 724.
Abstract
Purpose
The use of tolerogenic dendritic cells (TolDCs) to control exacerbated immune responses may be a prophylactic and therapeutic option for application in autoimmune and allergic conditions. The objective of this work was to evaluate the effects of TolDC administration in a mouse model of allergic airway inflammation caused by mite extract.
Methods
Mouse bone marrow-derived TolDCs were induced by incubation with granulocyte-macrophage colony-stimulating factor (GM-CSF) and dexamethasone, and then characterized by flow cytometry and cytokine production by enzyme-linked immunosorbent assay (ELISA). For the in vivo model of Blomia tropicalis-induced allergy, mice transplanted with antigen-pulsed TolDCs were sensitized intraperitoneally with B. tropicalis mite extract (BtE) adsorbed to aluminium hydroxide. After challenge by nasal administration of BtE, bronchoalveolar lavage fluid (BALF), lungs, spleen and serum were collected for analysis.
Results
Induction of TolDCs was efficiently achieved as shown by low expression of major histocompatibility complex (MHC) II, programmed death-ligand (PD-L) 2 and pro-inflammatory cytokine production, and up-regulation of interleukin (IL)-10, upon LPS stimulation in vitro. Transplantation of 1 or 2 doses of BtE-pulsed TolDCs reduced the number of inflammatory cells in BALF and lungs as well as mucus deposition. Moreover, compared to saline-injected controls, TolDC-treated mice showed lower serum levels of anti-BtE immunoglobulin E (IgE) antibodies as well as reduced Gata3 and IL-4 gene expression in the lungs and decreased IFN-γ levels in the supernatant of splenocyte cultures Transplantation of TolDCs increased the percentage of the regulatory T cells in the spleen and the lungs.
Asthma is a syndrome of recurrent respiratory symptoms greatly impacting on health care resources of people in all parts of the world with a high prevalence.1 In Brazil, the hospitalization admission rate due to asthma is 59.85 per 100,000 inhabitants (2012–2014), and such patients have a significantly increased risk of respiratory and all-cause mortality.2
The concept of allergic asthma equates to a group immune pathogenic and clinical characteristics such as T helper type 2 (Th2) responses and eosinophilic airway inflammation mediated by allergen-specific immunoglobulin E (IgE), leading to reversible airway obstruction, bronchoconstriction in association with airway remodeling and hyperresponsiveness.3 Asthma is triggered by various factors including viral respiratory infections, environmental allergens, pollution and climate changes.4 Mites are associated with allergic rhinitis and asthma, and a frequency of the Blomia tropicalis mite of 71.8% has been described in beds in the city of Salvador, Northeast of Brazil.5
There are many published reports regarding allergic disease therapies such as allergen-specific subcutaneous immunotherapy,6 immunotherapies using cytokines or bacterial products like adjuvants,7 corticosteroids,8 β2-agonists,9 inhibitors of the cellular immune response,1011 and targeting of Th2 cytokines.2 However, disease remission is not always achieved in a patient, especially considering all the broad asthma phenotypes.13 While corticosteroids are the most effective pharmacotherapies, they have the limitation of inhibiting the immune response to allergens through nonspecific mechanisms.914 Recently, the induction of immune tolerance has become an important strategy for the prevention and treatment of several diseases such as allergic diseases in which immune system dysregulation plays a crucial role.15
Dendritic cells (DCs) constitute an immunophenotipically and functionally heterogeneous population of professional antigen-presenting cells specialized in driving T-cell priming and differentiation,1617 which is established during the process of maturation.18 Antigen-presenting DCs in a state of partial maturation have the capacity to induce immunological tolerance.19 The potential to reprogram an immune response in an antigen-specific manner has made them an interesting target for immunotherapeutic strategies aimed at controlling inflammatory and autoimmune diseases. Tolerogenic DCs (TolDCs) have already been used prophylactically and therapeutically to prevent the development of allergic respiratory diseases in laboratory animals using ovalbumin as allergen.202122
In the present study, we investigated whether the injection of TolDCs prevents airway inflammation in a model of allergy induced by B. tropicalis mite extract (BtE).
Five- to 8-week-old female A strain mice were bred and maintained in the animal house of the Gonçalo Moniz Institute, Oswaldo Cruz Foundation, Salvador, Brazil. The current work was carried out in accordance with the Brazilian Federal Law on Animal Experimentation (Law 11794). The protocol was approved by the Ethics Committee for the Use of Animals in Research (IGM-FIOCRUZ, CEUA, license number 014/2012).
The method of production of bone marrow-derived DCs was adapted from a previously described protocol.18 Bone marrow cells from A strain mice were collected by flushing the femurs with RPMI medium (Sigma-Aldrich, St. Louis, MO, USA). The cells were then cultured in 75-cm2 flasks at a concentration of 106 nucleated cells/mL in RPMI medium supplemented with 100 mM pyruvate, 200 mM glutamine, 10 mM HEPES, 10% fetal bovine serum (FBS; Cripion, São Paulo, Brazil), 50–80 µg/mL gentamicin, 0.2% NaHCO3 and 30% culture supernatant of X-63 cell line (murine myeloma cells which produces granulocyte-macrophage colony-stimulating factor [GM-CSF]) at 37℃ in a 5% CO2 atmosphere. After 72 hours, the medium containing nonadherent cells was harvested and submitted to centrifugation, and the cell pellet was resuspended with fresh medium and replated. Dexamethasone (10−6 M; Decadron, Prodome Laboratory, Campinas, SP, Brazil) was added for additional 3 days of incubation. Cultures were then pulsed with BtE (100 µg of protein/mL medium) overnight on day 6 of cultivation. On day 7, TolDCs were activated with 1 µg/mL Escherichia coli lipopolysaccharide (LPS; Sigma-Aldrich) for 24 or 48 hours (Fig. 1A).
The phenotypic characterization of DCs was carried out by 3-color flow cytometry. Monoclonal antibody (mAb)-fluorochrome or biotin conjugates — fluorescein isothiocyanate (FITC) anti-CD11c mAb, phycoerythrin-cyanine anti-CD11b mAb, biotin anti-I-Ad mAb, biotin anti-programmed death-ligand (PD-L) 1, biotin anti-PD-L2, phycoerythrin (PE) anti-CD40 mAb, PE anti-CD80 mAb, PE anti-CD86 mAb — were purchased from eBioscience Inc. (San Jose, CA, USA). The cells were incubated with the conjugates or with the corresponding isotypes on ice for 20 minutes and washed twice with 0.15 M phosphate-buffered saline (PBS) at pH 7.2. The cells incubated with biotin-mAb conjugates were subsequently incubated with PE-avidin for 20 minutes. The cell suspensions were then washed once with PBS. For each sample, data from 100,000 cells is acquired. Fluorescence was measured using a FACS Calibur cytometer (Becton Dickinson, Heidelberg, Germany) and analyzed with Flow Jo version 10 (Treestar Inc., Ashland, OR, USA).
DCs were typically 50% to 69% CD11c+CD11b+ as published elsewhere.23 DCs were also tested for cytokine production after LPS stimulation. Cell-free supernatants from DCs and TolDCs were harvested 24 and 48 hours after LPS stimulation as described above, and stored at −20℃ until used for cytokine measurement. Interleukin (IL)-1β, IL-6, IL-10, IL-12 and tumor necrosis factor (TNF)-α concentrations were quantified by commercial enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go! kits in accordance with the manufacturer's instructions (eBioscience Inc.).
Four groups of A strain mice (n=7–8) were used: 1) naïve controls; 2) sensitized with BtE; 3) transplanted with one dose of TolDCs and sensitized with BtE; and 4) transplanted with 2 doses of TolDCs, sensitized with BtE. To induce tolerance to BtE, mice were injected intraperitoneally with 106 TolDCs, in 100 µL of saline 10 and/or 5 days before the first sensitizing exposure to BtE. Mice were sensitized with intraperitoneal injections of BtE containing 100 µg of protein adsorbed to 1.6 mg of aluminum hydroxide gel (Sigma-Aldrich), and a booster was injected 14 days later. Challenge was induced intranasally with 4 applications of a solution containing 10 µg of BtE in 25µL of saline starting 7 days after booster at 1-day intervals. The naïve control group received only saline injections. Mice were euthanized 24 hours after the last challenge.
The tracheas of the euthanized mice were cannulated, and the BALF was collected with 0.5 mL of PBS containing 1% of bovine serum albumin (PBS-BSA; Sigma-Aldrich). An aliquot of the BALF cells was washed by centrifugation (400 g for 5 minutes at 4℃), and the cell pellet was resuspended in PBS-BSA. Total cell counts were carried out in a Neubauer chamber. Differential cell counts were performed in a blinded manner by counting 200 cells in hematoxylin and eosin-stained cytospin preparations, using a light microscope (BX41 microscope; Olympus, Tokyo, Japan).
Splenocyte suspensions from mice of the different experimental groups were prepared in RPMI medium supplemented with 10% FBS and 50 µµg/mL gentamicin, and plated (106 cells/well) on 96-well plates, in triplicate, with or without stimulation with concanavalin A (Con A, 2 µg/mL; Sigma-Aldrich) or B. tropicalis antigen (100 µg/mL). After 48 hours of culture, cell-free supernatants were collected and kept at −80℃ until used for cytokine quantification.
The BALF and the splenocytes culture supernatants were stored at −70℃ until used. Cytokines were measured with BD CBA Mouse Th1/Th2/Th17 Cytokine Kit (BD Bioscience, San Jose, CA, USA). The kit was used for the simultaneous detection of mouse IL-2, IL-4, IL-6, interferon (IFN)-γ, TNF, IL-17A, and IL-10 in a single sample. The operations were performed according to the manufacturer's instructions. Beads coated with 7 specific capture antibodies were mixed. Subsequently, 50 µL of the mixed captured beads, 50 µL of the unknown sample or standard dilutions, and 50 µL of phycoerythrin (PE) detection reagent were added consecutively to each assay tube and incubated for 2 hours at room temperature in the dark. The samples were washed with 1 mL of wash buffer (200 g) for 5 minutes and centrifuged. The bead pellet was resuspended in 300 µL of buffer after discarding the supernatant. Samples were measured on the BD FACS Array Flow Cytometer and analyzed by FCAP Array™ Software (BD Bioscience). Individual cytokine concentrations were indicated by their fluorescent intensities. The theoretical limits of detection were 0.1 pg/mL for IL-2, 0.03 pg/mL for IL-4, 1.4 pg/mL for IL-6, 0.5 pg/mL for IFN-γ, 0.9 pg/mL for TNF, 0.8 pg/mL for IL-17A and 16.8 pg/mL for IL-10.
The right lobe of the lungs from each animal was removed for histological preparations. The lung was inflated via the tracheal cannula with 4% buffered formalin, fixed in the same solution, and embedded in paraffin. Lung sections were stained with hematoxylin and eosin for the quantification of inflammatory cells by optical microscopy. For each lung, 10 fields (400×) were analyzed per section, and data was used to calculate the mean number of cells per mm2. Mucus production was evaluated in alcian blue-stained sections. All images were digitized using a color digital video camera (CoolSnap cf) adapted to a BX41 microscope (Olympus), calibrated with a reference measurement slide, and analyzed using ImagePro program (version 6.1; Media Cybernetics, San Diego, CA, USA).
RNA was extracted from the lung tissue with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and its concentration was measured by photometry. A High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used to synthesize cDNA from 1 µg of RNA according to the manufacturer's recommendations. Transcript expression analysis was performed by Real-Time PCR using TaqMan Gene Expression Assay for Ptprc (Mm01293577), Il4 (Mm00445259_m1), and Gata3 (Mm00484683m1). All reactions were run on an ABI 7500 Real Time PCR System (Applied Biosystems) under standard thermal cycling conditions. A non-template control (NTC) and non-reverse transcription controls (No-RT) were also included. The samples were normalized with Gapdh (endogenous control-Mn99999915g1). The 2−ΔΔCt method was used to compare relative changes in gene expression.
Serum levels of total IgE and BtE-specific IgE were measured by ELISA. For the analysis of total IgE, 96-well flat-bottom plates were coated overnight with anti-mouse IgE monoclonal antibody (mAb) (BD PharMingen, San Jose, CA, USA) at 4℃. The plate was washed with PBS-T (PBS containing 0.05% Tween-20) 3 times, and nonspecific antigen-antibody reactions were blocked with 300 µL of PBS containing 3% BSA per well for 1 hour at room temperature. Serum samples diluted 1:4 in PBS containing 0.05% Tween and 5% segmented filamentous bacteria (SFB) were added to the 96-well plates along with purified mouse IgE isotype (BD PharMingen) used as a standard, and the plates were incubated for 3 hours at 4℃. For the analysis of BtE-specific IgE, 96-well microtitre plates were coated with BtE 100 µg/mL in coating buffer (0.05 M carbonate-bicarbonate) overnight at 4℃. The plates were washed 3 times with PBS-T and blocked with 5% FBS in PBS for 1 hour at 37℃. Serum samples diluted 1:4 in PBS containing 0.05% Tween and 5% SFB were added to BtE-coated plates and incubated overnight at 37℃. After washing, 100 µL of biotin-conjugated rat anti-mouse IgE mAb at 2 µg/mL (eBioscience Inc.) was added to each well and incubated for 1 hour at 37℃. After washing 3 times, the plates were then incubated with 100 µL of horseradish peroxidase (HRP)-conjugated secondary antibody (eBioscience Inc.) for 30 minutes at 37℃. The reactions were developed using 3,3′,5,5′-tetramethylbenzidine (TMB) (Moss Inc., Belfast, ME, USA) and were terminated by adding 1 N H2SO4. The optical density was measured using a microplate reader (Envision 2104 multilabel reader; Perkin Elmer, Wellesley, MA, USA) at 450 nm.
First, 10-µm lung sections were obtained in cryostat (Leica, Wetzlar, Germany) and were left at room temperature to dry for 1 hour. Next, the sections were fixed with 4% PFA for 15 minutes and washed 3 times with 0.01% PBS/triton X100. Sections were then incubated with protein block serum free (5 minutes, Dako, Santa Clara, CA, USA) to block nonspecific binding and were stained with the primary anti-Foxp3 antibody produced in rabbit (1:800;Abcam) and anti-CD3 produced in goat (1:400; Santa Cruz Biotechnology, Dallas, TX, USA) overnigth at 4℃. After that, the slides were washed with PBS 0.05% Tween 20 and 1×PBS and were incubated anti-goat IgG conjugated to Alexa Fluor 488 and anti-rabbit IgG conjugated to Alexa Fluor 568 (1:600; Molecular Probes, Carlsbad, CA, USA) for 1 hour. After incubation, the slides were washed with 0.05% Tween 20 PBS and 1×PBS, and were mounted with glass coverslips using the mounting medium Vectashield (Vector Laboratories, Inc., Burligame, CA, USA) containing 4′,6-Diamidino-2-phenylindole (DAPI) to label the nuclei. The images were obtained under a Fluoview 1000 confocal microscope (FV 1000; Olympus).
To evaluate the recruitment of Tregs induced by TolDCs treatment, the lung cells and splenocytes from animals were disrupted into individual cells. The cells (106) were stained with anti-CD4-APC and anti-CD25-FITC, or the corresponding isotypes, in accordance with the manufacturer's recommendations (eBioscience). After surface staining, the cells were permeabilized using a Cytofix/Cytoperm kit (eBioscience), and the cells were then stained with anti-Foxp3-PE (eBioscience). Fluorescence was measured using a FACS Fortessa cytometer (Becton Dickinson) equipped with BD FACSDiva 6.1 software (Becton Dickinson).
The normality of the data was determined by the Shapiro-Wilk normality test. In order to analyze differences among groups, the one-way analysis of variance test followed by the Newman Keuls test was used for parametric data and the Kruskal-Wallis test followed by Dunn's post-test was used for nonparametric data. To compare the means of the 2 groups, Mann-Whitney's U test for nonparametric data and Student's t test for parametric data were used. All results were considered statistically significant when P<0.05.
TolDCs were generated in vitro by adding dexamethasone on the third and sixth days of bone-marrow cell culture in the presence of GM-CSF, followed by activation with LPS.24 DCs were characterized as CD11c+CD11b+ cells, and after this protocol they constituted approximately 60% of the viable cells in culture (Fig. 1B). Double-positive cells were gated for posterior analysis of costimulatory molecules. The exposure of immature DCs to dexamethasone resulted in a semimature phenotype. Although only a small percentage of DCs expressed CD40 (4.5%), this percentage was 2.25-fold lower in TolDCs (2.0%, Fig. 1C). The down-regulation of CD80 (66% of the cells in TolDCs cultures and 96% in DCs cultures; Fig. 1D) was mainly due to a reduction in the CD80 high subpopulation from 42% to 13%. The expression of the costimulatory molecule CD86 was reduced to 15% of the cells in TolDCs cultures compared to 46% in DCs cultures (Fig. 1E). A higher percentage of cells expressing PD-L1 was observed in the TolDCs culture than in the DCs (52% vs 36%) (Fig. 1F). On the other hand, PD-L2 was strikingly down-regulated (from 42.0% to 5.6%) in the TolDCs (Fig. 1G). A reduction in major histocompatibility complex (MHC) II expression was observed (43.51% of the cells in TolDCs cultures and 90.51% in DCs cultures, Fig. 1H) mainly accounted for by the MHC II-high subpopulation (21.6% in the TolDCs and 51.3% in DCs).
We evaluated the cytokines produced by the dendritic cells upon LPS stimulation and found a lack of TolDC-mediated secretion of TNF-α, IL-12p70, and IL-1β, while the control DCs produced these 3 proinflammatory cytokines (Fig. 2A–C). Both DC preparations produced similar amounts of monocyte chemoattractant protein-1 (MCP-1) (Fig. 2D). Although IL-10 production was detected in both DC populations, the concentration of IL-10 was significantly higher in TolDCs than in control DCs 48 hours after LPS stimulation (Fig. 2E).
Collectively, these results confirm that DCs with an immunosuppressive phenotype were generated from bone marrow cells in the presence of dexamethasone during their differentiation process.
DCs generated in the presence of dexamethasone were used to induce immune tolerance to BtE hypersensitivity. One million TolDCs pulsed with BtE were injected intraperitoneally prior to the induction of airway inflammation (Fig. 3A). Mice sensitized and challenged with BtE had increased numbers of inflammatory cells in BALF compared to control mice sensitized and challenged with saline (Fig. 3B and C). The effects of TolDC treatment in lung inflammation were evaluated by comparing BALF cytology of TolDC-treated mice to that of mice treated with vehicle. The numbers of total cells and eosinophils in the BALF were significantly reduced after tolerization treatment (Fig. 3B and C), but we observed a small increase in the number of neutrophils and a reduction in the number of macrophages in the group that was injected with TolDC 1x (Fig. 3D). No significant difference was observed in the number of lymphocytes among the groups (Fig. 3D).
The levels of total and serum B. tropicalis-specific IgE antibodies in B. tropicalis-immunized mice treated with vehicle were higher compared to the control mice (Fig. 4A and B). A significant reduction in B. tropicalis-specific IgE antibodies was observed in mice treated with TolDCs in both cell doses tested compared to the saline-treated controls (Fig. 4A and B). Real-time PCR analysis showed an increased expression of Gata3 mRNA in the mouse lung tissue from the BtE-immunized group compared to the control group. Tolerance induction by DC transplantation resulted in a reduction in gene expression of Gata3 mRNA in the lung tissue compared to the BtE-immunized group, reaching the levels similar to those of the control group (Fig. 4C). The gene expression of IL-4, a Th2 cytokine, was increased in the lung tissue in the BtE-immunized group compared to the control mice. Pretreatment with TolDCs 1x caused a statistically significant reduction in IL-4 gene expression compared to BtE-immunized mice (Fig. 4D). The assessment of IL-4, IL-5, IL-6, IL-10, IL-13, IL-17A, TNF-α, and IFN-γ in BALF showed low levels in the experimental groups, with no statistically significant differences (data not shown). Additionally, we evaluated cytokine production by spleen cells stimulated with Con A or BtE. Upon in vitro stimulation, spleen cells from mice transplanted with TolDCs had a significant reduction in IFN-γ production compared to the BtE-immunized group (Fig. 4E). The levels of IL-2, IL-4, and IL-10 were low and did not present statistically significant differences among the experimental groups (data not shown).
Lung sections from B. tropicalis-challenged mice stained with H&E showed an intense cell infiltrate of polymorphonuclear cells when compared to naïve mice (Fig. 5A and B). Mice treated with 2 doses of TolDCs had a reduced number of inflammatory cells, whereas the group treated with only 1 dose did not differ from the saline-treated controls (Fig. 5C–E). Gene expression analysis of the leukocyte common antigen (Ptprc/CD45) in the lungs showed a strong up-regulation in the saline-treated BtE-challenged group compared to the naïve controls, whereas pretreatment with TolDCs significantly reduced the expression of this receptor (Fig. 5F). Finally, alcian blue staining used for the analysis of mucus production demonstrated an intense mucus deposition in lung sections of BtE-immunized mice when compared to naïve mice (Fig. 6A, B and E). In contrast, both protocols of tolerization with TolDCs reduced the production of mucus as shown with morphometrical analysis (Fig. 6C–E).
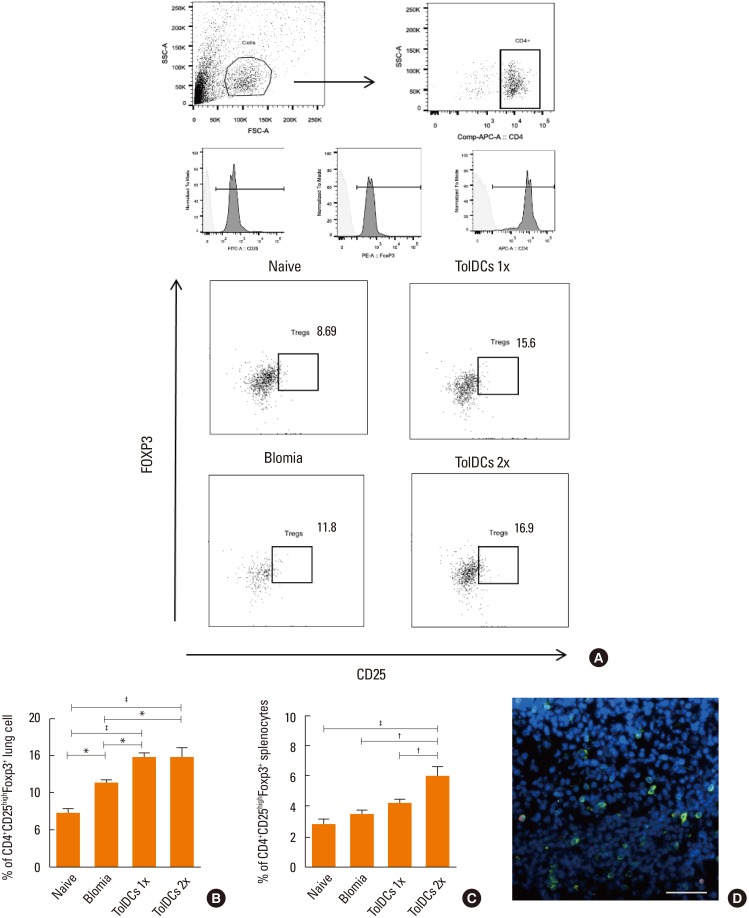
To evaluate whether the presence of TolDCs were able to promote the differentiation of Tregs as an induction mechanism for immunological tolerance, flow cytometric analysis of splenocytes and lung mononuclear cells from individual mice was performed 24 hours after the last BtE challenge. An increase in the number of Foxp3+ T cells was found in the groups treated with TolDCs in both cell doses in the lungs (Fig. 7A and B) and after 2 doses in the spleen (Fig. 7C), compared to the control groups. Immunostaining for CD3 and Foxp3 confirmed the presence of Tregs in the lung sections of TolDC-treated mice (Fig. 7D).
Airway inflammation is strongly associated with worsening asthma symptoms and is mediated by factors that involve the interaction between the pulmonary epithelium and DCs. The use of bone marrow-derived TolDCs to prevent allergy development, which has been increased over the last decades,525 was studied for B. tropicalis for the first time in this paper. Importantly, we demonstrated the functional activity of TolDCs pulsed with BtE inducing a protective response in an allergic airway inflammation murine model.
In the present work, murine CD11c+CD11b+ DCs with a tolerogenic profile were successfully generated in vitro in the presence of GM-CSF and dexamethasone as previously described.2627 It has been shown that resident lung CD11b+ DCs are crucial for the initiation of Th2 responses.2829 Thus, the modulation of CD11b+ DCs to inhibit T-cell priming to a Th2 profile may be an advantageous tool for allergy intervention. DCs generated in the presence of corticosteroids and activated by LPS are characterized by increased expression of inhibitory molecules and production of suppressor cytokines associated with reduction in pro-inflammatory cytokines.30 However, there are many approaches to generating DCs with immunosuppressive activity.18233033 These cells may present distinct phenotypic and functional characteristics according to the stimulus received.
The DCs produced had low expressions of MHC II and the costimulatory molecules CD80, CD86, and CD40, a well as showed a lack of production of the proinflammatory cytokines TNF-α, IL-12p70, and IL-1β. These features, associated with increased expression of PD-L1 and IL-10 production are characteristic of an immunosuppressive profile.3132 Recent studies demonstrated that the lack of IL-12p70 production is a key feature of tolerogenic DCs, which is important for their regulatory action. The replacement of this cytokine can prevent anergy of T cells and its absence, together the increased IL-10 production, which is considered a quality control criterion for therapeutic DC preparations.33 Although we observed no difference in IL-10 production within 24 hours after LPS activation, the difference between mature cells and TolDCs was clear after 48 hours, indicating the tolerogenic potential of these cells. Lee et al.34 compared the effect of different stimuli (rapamycin, vitamin D3, IL-10, dexamethasone and minocycline) on DC generation with regulatory properties and observed that the level of IL-10 produced varies according to the stimulus. Further work shows that the culture time of these cells also influences the level of IL-10 produced.182635 Interestingly, a substantial reduction in PD-L2 expression was observed in the TolDCs produced here. The role of PD-L1 in allergy has been controversial, although some studies have shown that PD-L2 blockade is related to increased bronchial hyperreactivity (airway hyperresponsiveness, AHR), eosinophilia and pulmonary inflammation,3637 while others have demonstrated an increased expression in PD-L2 in the lung of sensitized mice3839 and a reduction in the production of IL-12p70 and AHR when PD-L2 expression is modulated.39 Our results suggest that in an allergy context, low PD-L2 expression in TolDCs is sufficient to indicate those cells as therapeutic agents.
Pretreatment with TolDCs reduced the recruitment of total cells and eosinophils to the BALF, lung inflammation and mucus production. These effects are probably associated with a decreased Th2 response, since TolDCs modulated the gene expression of Gata3 and IL-4 that are key factors promoting Th2 responses.14
Although the participation of cytokines in allergic response is well established in the literature, low levels of cytokines were detected in the BALF of mice, with no differences among the groups, through the 2 techniques ELISA and CBA. This data was not expected once we used to find increased levels of Th2 cytokines in BALF in the ovalbumin-induced BALB/c mouse model.4041 Thus, we attribute the low production of cytokines in BALF to differences in the experimental model used in this study. Additionally, serum IgE production, which is IL-4-dependent, was reduced in TolDC-treated mice upon challenge with BtE. Antigen-specific IgE antibodies bind to Fcε receptors in eosinophils, leading to the release of mediators that induce airway remodeling, mucus production and attraction of inflammatory cells to the lung.442434445 Although some therapeutic protocols for experimental asthma have presented better results after booster doses2246 in the protocol used in this study, no difference in allergic modulation between the uses of only 1 and 2 inoculations of TolDCs was observed. This result may be explained by the fact that our cells were maintained longer in the presence of dexamethasone as the treatment for 1 hour is related to an in vivo instability of these cells,47 while treatment for 48 hours produced better results in terms of inhibiting IFN-γ production by effector lymphocytes than a 6-hour treatment.48 This effect in down-regulating IFN-γ was also observed after injection of TolDCs generated here, favoring the hypothesis that action of these cells is mainly systemic, affecting immune responses in peripheral lymphoid organs, since they were transplanted before allergy induction. In fact, Floderer et al.26 demonstrated that the subcutaneous immunization of wild-type mice with DCs affects the cytokine microenvironment in the spleen, causing T-cell phenotype reprogramming.
The capacity to produce IL-10, together with the low costimulatory phenotype, observed in this work was consistent with the view that TolDCs could induce Tregs, characterized by CD4+CD25+Foxp3+ cells49 as shown by other studies.47 In fact, we observed that Foxp3+ cells were recruited to the spleen and the lungs of TolDC-treated mice, suggesting that the mechanism for immunological tolerance induced by TolDCs pulsed with BtE could include the participation of Tregs. The prevention of allergic feature development by CD4+CD25+Foxp3+ cells was previously demonstrated in an ovalbumin-induced model50 and in an ex vivo experiment with cells obtained from atopic donors,19 both using DCs induced by IL-10.
In conclusion, tolerogenic DCs are promising immunotherapeutic tools for immunologically mediated pathologies. However, there is no consensus in the literature regarding the most appropriate methodology that can be used for a particular disease. The work described here shows that bone marrow-derived DCs generated in the presence of dexamethasone and stimulated in vitro with BtE can inhibit the development of a Th2 allergic response as a prophylactic therapy. This methodology deserves to be further investigated with the aim of specifically inhibiting an ongoing allergic response over a prolonged period of time.
ACKNOWLEDGMENTS
The authors thank Dr. Bruno Solano de Freitas Souza and Dr. Kyan James Allahdadi for critically reading the manuscript.
References
1. Ferrando M, Bagnasco D, Varricchi G, Bernardi S, Bragantini A, Passalacqua G, et al. Personalized medicine in allergy. Allergy Asthma Immunol Res. 2017; 9:15–24. PMID: 27826958.

2. Comaru T, Pitrez PM, Friedrich FO, Silveira VD, Pinto LA. Free asthma medications reduces hospital admissions in Brazil (free asthma drugs reduces hospitalizations in Brazil). Respir Med. 2016; 121:21–25. PMID: 27888987.
3. Mizutani N, Nabe T, Yoshino S. Interleukin-33 and alveolar macrophages contribute to the mechanisms underlying the exacerbation of IgE-mediated airway inflammation and remodelling in mice. Immunology. 2013; 139:205–218. PMID: 23323935.

4. Cabral AL, Sousa AW, Mendes FA, de Carvalho CR. Phenotypes of asthma in low-income children and adolescents: cluster analysis. J Bras Pneumol. 2017; 43:44–50. PMID: 28125150.

5. Baqueiro T, Carvalho FM, Rios CF, dos Santos NM, Alcântara-Neves NM. Dust mite species and allergen concentrations in beds of individuals belonging to different urban socioeconomic groups in Brazil. J Asthma. 2006; 43:101–105. PMID: 16517425.

6. Lee JH, Kim SC, Choi H, Jung CG, Ban GY, Shin YS, et al. A retrospective study of clinical response predictors in subcutaneous allergen immunotherapy with house dust mites for allergic rhinitis. Allergy Asthma Immunol Res. 2018; 10:18–24. PMID: 29178674.

7. Walker C, Zuany-Amorim C. New trends in immunotherapy to prevent atopic diseases. Trends Pharmacol Sci. 2001; 22:84–90. PMID: 11166852.

8. Kandeel M, Balaha M, Inagaki N, Kitade Y. Current and future asthma therapies. Drugs Today (Barc). 2013; 49:325–339. PMID: 23724412.

9. Page CP, Spina D. Beta2-agonists and bronchial hyperresponsiveness. Clin Rev Allergy Immunol. 2006; 31:143–162. PMID: 17085790.
10. Kon OM, Sihra BS, Loh LC, Barkans J, Compton CH, Barnes NC, et al. The effects of an anti-CD4 monoclonal antibody, keliximab, on peripheral blood CD4+ T-cells in asthma. Eur Respir J. 2001; 18:45–52. PMID: 11510804.

11. Gervais FG, Sawyer N, Stocco R, Hamel M, Krawczyk C, Sillaots S, et al. Pharmacological characterization of MK-7246, a potent and selective CRTH2 (chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells) antagonist. Mol Pharmacol. 2011; 79:69–76. PMID: 20943773.

12. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659. PMID: 22901886.

13. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18:716–725. PMID: 22561835.

14. Maneechotesuwan K, Yao X, Ito K, Jazrawi E, Usmani OS, Adcock IM, et al. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med. 2009; 6:e1000076. PMID: 19436703.

15. Akdis CA. Allergy and hypersensitivity: mechanisms of allergic disease. Curr Opin Immunol. 2006; 18:718–726. PMID: 17029937.
16. Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973; 137:1142–1162. PMID: 4573839.
17. Kushwah R, Hu J. Dendritic cell apoptosis: regulation of tolerance versus immunity. J Immunol. 2010; 185:795–802. PMID: 20601611.

18. Ureta G, Osorio F, Morales J, Rosemblatt M, Bono MR, Fierro JA. Generation of dendritic cells with regulatory properties. Transplant Proc. 2007; 39:633–637. PMID: 17445563.

19. Li X, Yang A, Huang H, Zhang X, Town J, Davis B, et al. Induction of type 2 T helper cell allergen tolerance by IL-10-differentiated regulatory dendritic cells. Am J Respir Cell Mol Biol. 2010; 42:190–199. PMID: 19372244.

20. Zhang-Hoover J, Finn P, Stein-Streilein J. Modulation of ovalbumin-induced airway inflammation and hyperreactivity by tolerogenic APC. J Immunol. 2005; 175:7117–7124. PMID: 16301614.

21. Koya T, Matsuda H, Takeda K, Matsubara S, Miyahara N, Balhorn A, et al. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J Allergy Clin Immunol. 2007; 119:1241–1250. PMID: 17353041.

22. Nayyar A, Dawicki W, Huang H, Lu M, Zhang X, Gordon JR. Induction of prolonged asthma tolerance by IL-10-differentiated dendritic cells: differential impact on airway hyperresponsiveness and the Th2 immunoinflammatory response. J Immunol. 2012; 189:72–79. PMID: 22634620.

23. Pedersen AE, Gad M, Kristensen NN, Haase C, Nielsen CH, Claesson MH. Tolerogenic dendritic cells pulsed with enterobacterial extract suppress development of colitis in the severe combined immunodeficiency transfer model. Immunology. 2007; 121:526–532. PMID: 17428312.

24. Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010; 207:2097–2111. PMID: 20819925.

25. Juliá-Serdá G, Cabrera-Navarro P, Acosta-Fernández O, Martín-Pérez P, García-Bello MA, Antó-Boqué J. Prevalence of sensitization to Blomia tropicalis among young adults in a temperate climate. J Asthma. 2012; 49:349–354. PMID: 22486531.
26. Floderer M, Prchal-Murphy M, Vizzardelli C. Dendritic cell-secreted lipocalin2 induces CD8+ T-cell apoptosis, contributes to T-cell priming and leads to a TH1 phenotype. PLoS One. 2014; 9:e101881. PMID: 25010215.

27. Matyszak MK, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000; 30:1233–1242. PMID: 10760813.

28. Zhou Q, Ho AW, Schlitzer A, Tang Y, Wong KH, Wong FH, et al. GM-CSF–licened CD11b + lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis. J Immunol. 2014; 193:496–509. PMID: 24943219.
29. van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005; 201:981–991. PMID: 15781587.
30. Svajger U, Rozman P. Tolerogenic dendritic cells: molecular and cellular mechanisms in transplantation. J Leukoc Biol. 2014; 95:53–69. PMID: 24108704.

31. Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front Immunol. 2013; 4:82. PMID: 23565116.

32. Hammer GE, Ma A. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Annu Rev Immunol. 2013; 31:743–791. PMID: 23330953.

33. Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, et al. ‘Alternatively activated’ dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006; 177:5868–5877. PMID: 17056511.

34. Lee JH, Park CS, Jang S, Kim JW, Kim SH, Song S, et al. Tolerogenic dendritic cells are efficiently generated using minocycline and dexamethasone. Sci Rep. 2017; 7:15087. PMID: 29118423.

35. Kvistborg P, Boegha M, Pedersen AW, Claesson MH, Zocca MB. Fast generation of dendritic cells. Cell Immunol. 2009; 260:56–62. PMID: 19818956.

36. Matsumoto K, Fukuyama S, Eguchi-Tsuda M, Nakano T, Matsumoto T, Matsumura M, et al. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun. 2008; 365:170–175. PMID: 17981145.

37. Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010; 3:81–91. PMID: 19741598.

38. Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol. 2004; 172:2530–2541. PMID: 14764726.
39. Lewkowich IP, Lajoie S, Stoffers SL, Suzuki Y, Richgels PK, Dienger K, et al. PD-L2 modulates asthma severity by directly decreasing dendritic cell IL-12 production. Mucosal Immunol. 2013; 6:728–739. PMID: 23149662.

40. Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Agra MF, Nunes XP, Giulietti AM, et al. Effects of umbelliferone in a murine model of allergic airway inflammation. Eur J Pharmacol. 2009; 609:126–131. PMID: 19289114.

41. Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Lúcio AS, Almeida JR, de Queiroz LP, et al. The triterpenoid lupeol attenuates allergic airway inflammation in a murine model. Int Immunopharmacol. 2008; 8:1216–1221. PMID: 18602067.

42. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006; 6:761–771. PMID: 16998509.

43. Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol. 2007; 142:265–273. PMID: 17124428.

44. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012; 18:673–683. PMID: 22561831.

45. Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE. A new look at the pathogenesis of asthma. Clin Sci (Lond). 2009; 118:439–450. PMID: 20025610.

46. Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009; 39:3147–3159. PMID: 19688742.
47. Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clin Immunol. 2012; 142:332–342. PMID: 22225835.
48. Wojas-Krawczyk K, Krawczyk P, Buczkowski J, Walkowska A, Jankowska O, Czekajska-Chehab E, et al. Immunotherapy of lung adenocarcinoma patient with Peptide-pulsed dendritic cells: a case report. Arch Immunol Ther Exp (Warsz). 2012; 60:69–77. PMID: 22143160.

49. Kim YJ, Kim HJ, Jeong SK, Lee SH, Kang MJ, Yu HS, et al. A novel synthetic mycolic acid inhibits bronchial hyperresponsiveness and allergic inflammation in a mouse model of asthma. Allergy Asthma Immunol Res. 2014; 6:83–88. PMID: 24404398.

50. Henry E, Desmet CJ, Garzé V, Fiévez L, Bedoret D, Heirman C, et al. Dendritic cells genetically engineered to express IL-10 induce long-lasting antigen-specific tolerance in experimental asthma. J Immunol. 2008; 181:7230–7242. PMID: 18981145.

Fig. 1
Immunophenotype of DCs differentiated from bone marrow cells in the presence of dexamethasone. (A) Protocol for the generation of tolerogenic DCs from A strain mouse bone marrow. Control cells were cultured in differentiation medium and stimulated with LPS in the absence of dexamethasone. (B–H) Cells were stained with antibodies against surface markers as indicated. Debris and dead cells were excluded on the basis of forward-scatter and side-scatter. (B) Representative dot plots depicting the percentages of CD11c+CD11b+ cells. (C-H) Histograms of CD40 (C), CD80 (D), CD86 (E), PD-L1 (F), PD-L2 (G), and MHC II (H) cells gated on CD11c+CD11b+ double positive cells. DCs are represented by filled gray histograms and TolDCs by black lines; isotype controls are shown by dotted gray lines. Results are representative of 3 independent experiments. DC, dendritic cell; LPS, lipopolysaccharide; PD-L, programmed death-ligand; MHC, major histocompatibility complex; TolDC, tolerogenic dendritic cell.
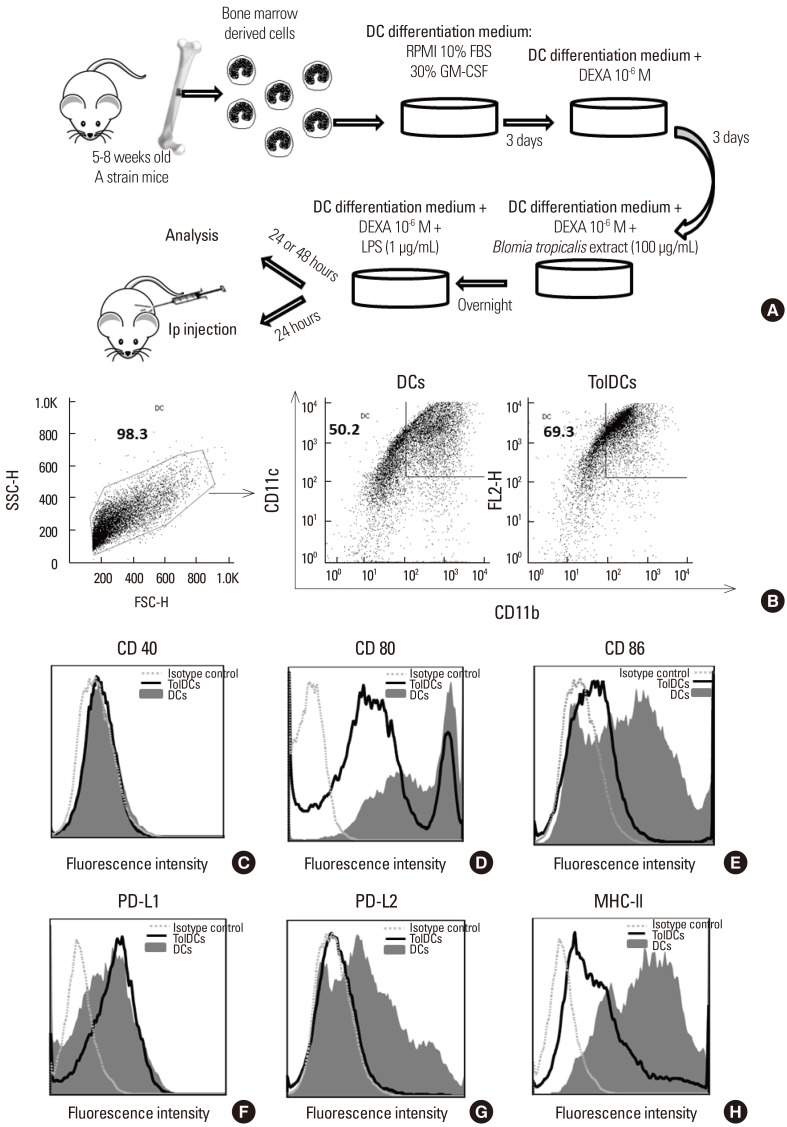
Fig. 2
Anti-inflammatory cytokine production profile of DCs differentiated from bone marrow cells in the presence of dexamethasone. Control cells were left to differentiate in the absence of dexamethasone. The supernatants from cultures were harvested 24 hours after activation with lipopolysaccharide for TNF-α (A), IL-12p70 (B), IL-1β (C) and MCP-1 (D), and the measurement of IL-10 (E) was performed 48 hours after stimuli with LPS. Cytokines were quantified by sandwich ELISA. The data represented is the median with a range of 4 (TNF-α, IL-12p70 and IL-1β), 7 (MCP-1) and 3 (IL-10), independent of experiments. DC, dendritic cell; TNF, tumor necrosis factor; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; LPS, lipopolysaccharide; ELISA, enzyme-linked immunosorbent assay. *P<0.05 (Mann-Whitney's U test).
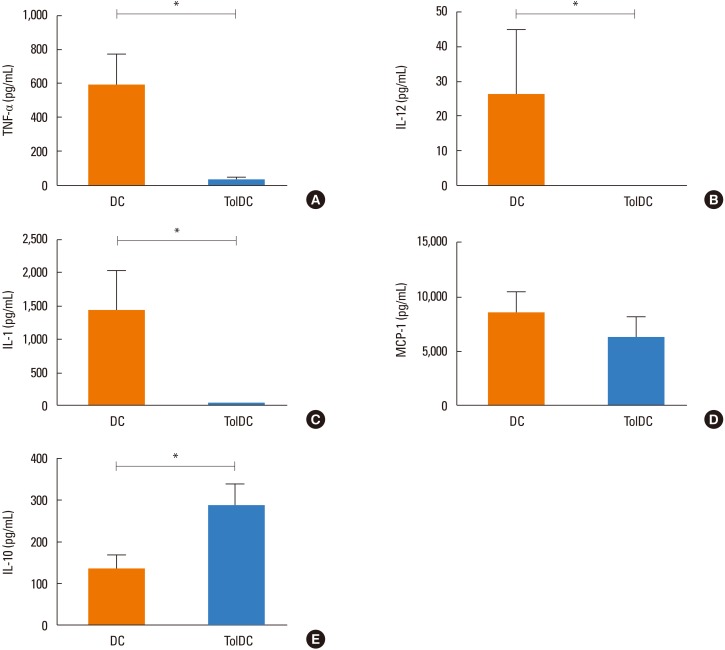
Fig. 3
Evaluation of Th2 parameters by dexamethasone-induced tolerogenic DCs. Experimental protocol of TolDC therapy in a mouse model of allergy to B. tropicalis (A). Mice were pre-treated with 1 or 2 doses of TolDCs prior to sensitization with BtE. Mice were euthanized 24 hours after the last challenge with BtE. The cellularity in BALF from naïve or Blomia-challenged mice, treated with 1 (TolDCs 1×) or 2 (TolDCs 2×) doses of TolDCs was evaluated. Total cell number (B), number of eosinophils in 200 cells (C) and differential (D) in BALF. Values are expressed as mean±SEM of 5–8 mice per group, in 1 of the 5 experiments performed. Th2, T helper type 2; DC, dendritic cell; TolDC, tolerogenic dendritic cell; BtE, B. tropicalis extract; BALF, bronchoalveolar lavage fluid; SEM, standard error of the mean. *P<0.05; †P<0.01; ‡P<0.001; and §P<0.05 compared to the naïve and TolDC 2x groups (B and C, Dunn's multiple comparison test; D, Newman-Keuls multiple comparison test).
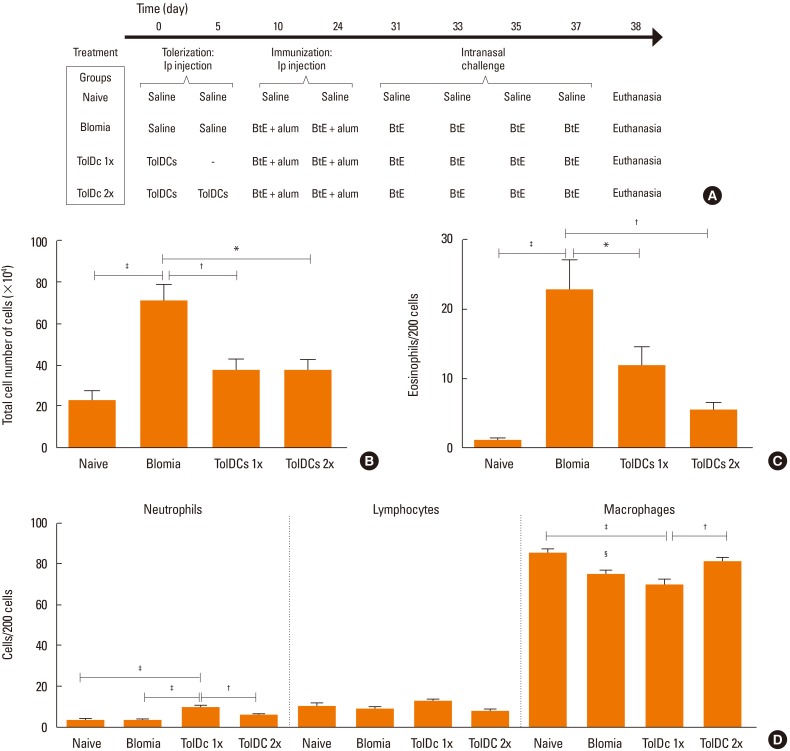
Fig. 4
Immunomodulation by TolDCs. Serum levels of total IgE (a) and BtE-specific IgE (B) were measured by ELISA. Relative expression to Gapdh of Gata3 (C) and IL-4 (D) of lung fragments of A strain mice treated with TolDCs previously to allergy induction, by Real-Time PCR. Levels of IFN-γ in the supernatant of splenocyte cultures were evaluated using CBA assay (E). Values are expressed as mean±SEM of 5–8 mice per group, in 1 of the 5 experiments performed. TolDC, tolerogenic dendritic cell; BtE, B. tropicalis mite extract; IgE, immunoglobulin E; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; IFN, interferon; CBA, cytometric bead array; SEM, standard error of the mean. *P<0.05; †P<0.01; ‡P<0.001 (Newman-Keuls multiple comparison test).
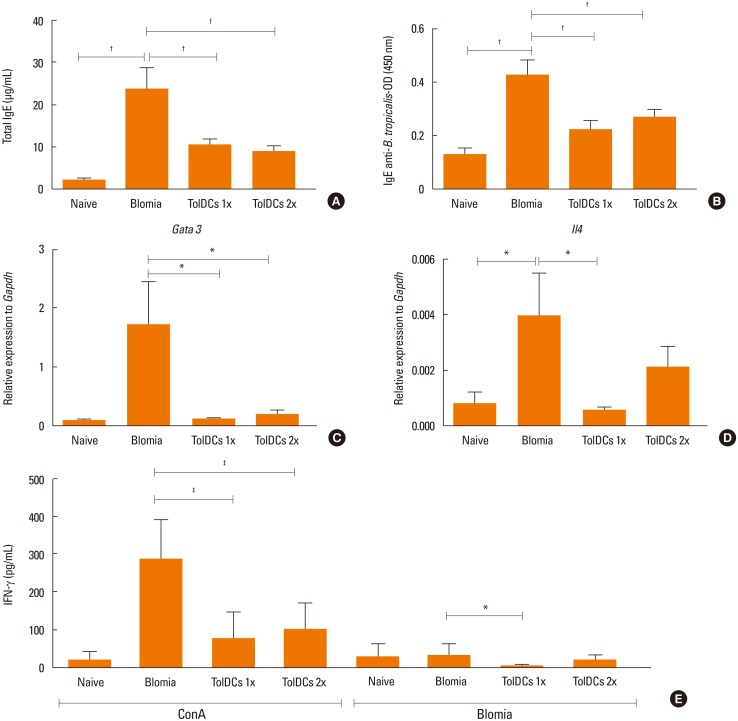
Fig. 5
Airway inflammation of TolDC-treated mice. The right lobe sections of the lungs were stained with H&E for the quantification of inflammatory cells by optical microscopy. For each of the lung 10 fields (400×) were analyzed per section, and the data used to calculate the mean number of cells per mm2. Scale bar, 50 µm. (A–D) Representative hematoxylin and eosin-stained sections of the lungs of A strain mice. (A) Normal tissue of an untreated animal. (B) Inflammatory infiltrate with a predominance of polymorphonuclear around bronchi and arterioles in an animal from the positive control group (Blomia). (C and D) Reduced lung perivascular infiltration by polymorphonuclear cells, in a Blomia-sensitized animal pre-treated with TolDCs 1× and TolDCs 2×, respectively. (E) Infiltration of inflammatory cells per mm2. (F) Relative expression to Gapdh of Ptprc. The data is representative of 3 independent experiments. TolDC, tolerogenic dendritic cell. *P<0.05; †P<0.01; ‡P<0.001 (Newman-Keuls multiple comparison test).
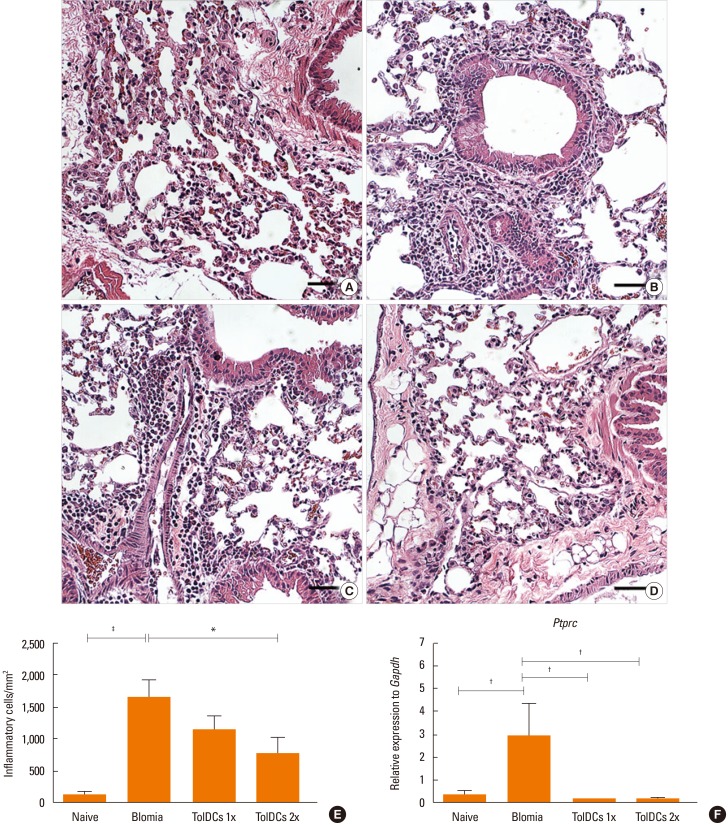
Fig. 6
Mucus analysis of lungs from allergic and tolerized mice. Lung sections of (A) control and (B) allergic mice treated with vehicle, (C) tolerized with TolDCs 1× or (D) tolerized with TolDCs 2×. Narrow arrows indicate areas of alcian blue+ cells (original magnification ×400). Scale bar, 50 µm. (E) Quantification of mucus production on alcian blue-stained lung sections. The area of alcian blue staining was estimated by morphometric analysis. Data is expressed as means±SEM of 5–8 mice per group, in 1 of the 2 experiments performed. TolDC, tolerogenic dendritic cell; SEM, standard error of the mean. *P<0.001 compared to vehicle-treated mice.
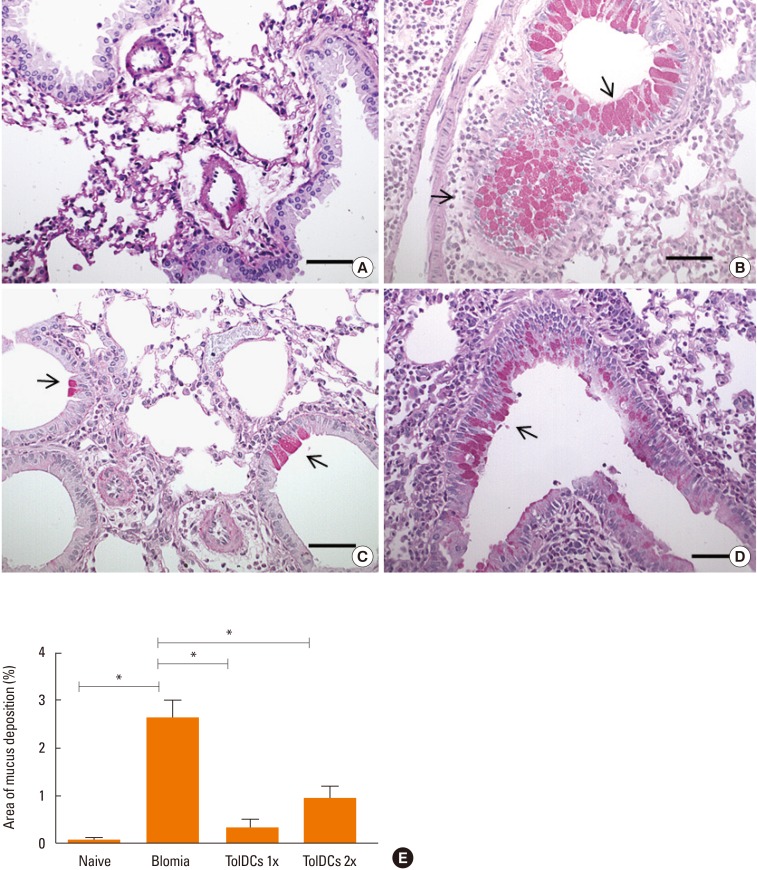
Fig. 7
TolDC treatment recruits Tregs to the lung and the spleen. The lung and spleen cell preparations obtained from mice of each experimental group were stained with anti-CD4-APC and anti-CD25-FITC, and after permeabilization the cells were stained with anti-Foxp3-PE. (A) Gating on CD4+ T cells, and representative dot plots of lung Tregs (CD4+CD25highFoxp3+) and isotype controls. (B) Quantification of lung Tregs (CD4+CD25highFoxp3+) relative to total CD4+ T cells. (C) CD4+CD25highFoxp3+ splenocytes. (D) Confocal microscopy images showing CD3 (green), Foxp3 (red), and nuclei stained with DAPI (blue) in the lung tissue of TolDC-treated mice. Values are expressed as means±SEM of 5–7 mice per group. TolDC, tolerogenic dendritic cell; Treg, regulatory T cell; FITC, fluorescein isothiocyanate; DAPI, 4′,6-Diamidino-2-phenylindole; SEM, standard error of the mean. *P<0.05; †P<0.01; ‡P<0.001 (Bonferroni's multiple comparison test).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download