1. Bousquet J, Van Cauwenberge P, Khaltaev N. Aria Workshop Group. World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001; 108:S147–S334. PMID:
11707753.

2. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010; 126:466–476. PMID:
20816182.
3. Roberts G, Xatzipsalti M, Borrego LM, Custovic A, Halken S, Hellings PW, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013; 68:1102–1116. PMID:
23952296.

4. Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015; 152:S1–S43.
5. Ellis AK, Soliman M, Steacy L, Boulay ME, Boulet LP, Keith PK, et al. The Allergic Rhinitis - Clinical Investigator Collaborative (AR-CIC): nasal allergen challenge protocol optimization for studying AR pathophysiology and evaluating novel therapies. Allergy Asthma Clin Immunol. 2015; 11:16. PMID:
25945101.

6. Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008; 122:S1–S84. PMID:
18662584.

7. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63(Suppl 86):8–160. PMID:
18331513.
8. Okubo K, Kurono Y, Fujieda S, Ogino S, Uchio E, Odajima H, et al. Japanese guideline for allergic rhinitis 2014. Allergol Int. 2014; 63:357–375.

9. Walls RS, Heddle RJ, Tang ML, Basger BJ, Solley GO, Yeo GT. Optimising the management of allergic rhinitis: an Australian perspective. Med J Aust. 2005; 182:28–33. PMID:
15651945.

10. Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guidelines for diagnosis and treatment of allergic rhinitis (2009, Wuyishan). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009; 44:977–978. PMID:
20193608.
11. Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016; 51:6–24. PMID:
26791765.
12. Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Subspecialty Group of Rhinology and Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Editorial Board of Chinese Journal of Pediatrics. Guidelines for diagnosis and treatment of pediatric allergic rhinitis (2010, Chongqing). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 46:7–8. PMID:
21429322.
13. Long A, McFadden C, DeVine D, Chew P, Kupelnick B, Lau J. Management of allergic and nonallergic rhinitis. Evid Rep Technol Assess (Summ). 2002; 1–6.
14. Yorgancioğlu A, Kalayci O, Kalyoncu AF, Khaltaev N, Bousquet J. Allergic rhinitis and its impact on asthma update (ARIA 2008). The Turkish perspective. Tuberk Toraks. 2008; 56:224–231. PMID:
18701986.
15. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004; 24:758–764. PMID:
15516669.

16. Bachert C, van Cauwenberge P, Olbrecht J, van Schoor J. Prevalence, classification and perception of allergic and nonallergic rhinitis in Belgium. Allergy. 2006; 61:693–698. PMID:
16677237.

17. Nathan RA, Meltzer EO, Derebery J, Campbell UB, Stang PE, Corrao MA, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008; 29:600–608. PMID:
19173786.

18. Katelaris CH, Lee BW, Potter PC, Maspero JF, Cingi C, Lopatin A, et al. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy. 2012; 42:186–207. PMID:
22092947.

19. Choi BC, McQueen DV, Puska P, Douglas KA, Ackland M, Campostrini S, et al. Enhancing global capacity in the surveillance, prevention, and control of chronic diseases: seven themes to consider and build upon. J Epidemiol Community Health. 2008; 62:391–397. PMID:
18413450.

20. Zhang L, Han D, Huang D, Wu Y, Dong Z, Xu G, et al. Prevalence of self-reported allergic rhinitis in eleven major cities in china. Int Arch Allergy Immunol. 2009; 149:47–57. PMID:
19033732.

21. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995; 8:483–491. PMID:
7789502.

22. Weiland SK, Björkstén B, Brunekreef B, Cookson WO, von Mutius E, Strachan DP, et al. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. Eur Respir J. 2004; 24:406–412. PMID:
15358699.

23. Ellwood P, Asher MI, Beasley R, Clayton TO, Stewart AW. ISAAC Steering Committee. The international study of asthma and allergies in childhood (ISAAC): phase three rationale and methods. Int J Tuberc Lung Dis. 2005; 9:10–16. PMID:
15675544.
24. Li F, Zhou Y, Li S, Jiang F, Jin X, Yan C, et al. Prevalence and risk factors of childhood allergic diseases in eight metropolitan cities in China: a multicenter study. BMC Public Health. 2011; 11:437. PMID:
21645361.

25. Zhang Y, Zhang L. Prevalence of allergic rhinitis in china. Allergy Asthma Immunol Res. 2014; 6:105–113. PMID:
24587945.

26. Wang XD, Zheng M, Lou HF, Wang CS, Zhang Y, Bo MY, et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016; 71:1170–1180. PMID:
26948849.

27. Zheng M, Wang X, Bo M, Wang K, Zhao Y, He F, et al. Prevalence of allergic rhinitis among adults in urban and rural areas of china: a population-based cross-sectional survey. Allergy Asthma Immunol Res. 2015; 7:148–157. PMID:
25729622.

28. Zhang YM, Zhang J, Liu SL, Zhang X, Yang SN, Gao J, et al. Prevalence and associated risk factors of allergic rhinitis in preschool children in Beijing. Laryngoscope. 2013; 123:28–35. PMID:
23280940.

29. Chen J, Zhao Y, Li B, Zhang Q, Wan L, Liu J, et al. A multicenter study of the clinical features of allergic rhinitis in central China. Am J Rhinol Allergy. 2014; 28:392–396. PMID:
25198025.

30. Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015; 372:456–463. PMID:
25629743.
31. Han DM, Zhang L, Huang D, Wu YF, Dong Z, Xu G, et al. Self-reported prevalence of allergic rhinitis in eleven cities in China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007; 42:378–384. PMID:
17629011.
32. Zhang L, Jin T, Han DM. Allergic rhinoconjunctivitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012; 47:173–176. PMID:
22455827.
33. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012; 1–298. 3 p preceding table of contents.
34. Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ, Zhu DD, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015; 70:533–539. PMID:
25631304.
35. Fu QL, Ma JX, Ou CQ, Guo C, Shen SQ, Xu G, et al. Influence of self-reported chronic rhinosinusitis on health-related quality of life: a population-based survey. PLoS One. 2015; 10:e0126881. PMID:
25978550.

36. Bousquet J, Schunemann HJ, Fonseca J, Samolinski B, Bachert C, Canonica GW, et al. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy. 2015; 70:1372–1392. PMID:
26148220.
37. Lai K, Chen R, Lin J, Huang K, Shen H, Kong L, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. 2013; 143:613–620. PMID:
23238526.

38. Lack G, Caulfield H, Penagos M. The link between otitis media with effusion and allergy: a potential role for intranasal corticosteroids. Pediatr Allergy Immunol. 2011; 22:258–266. PMID:
21457332.

39. Spieksma FT, Dieges PH. The history of the finding of the house dust mite. J Allergy Clin Immunol. 2004; 113:573–576. PMID:
15007368.

40. Stewart GA. Dust mite allergens. Clin Rev Allergy Immunol. 1995; 13:135–150. PMID:
7489260.

41. Fernández-Caldas E, Puerta L, Caraballo L, Lockey RF. Mite allergens. Clin Allergy Immunol. 2008; 21:161–182. PMID:
18828504.

42. Chan TF, Ji KM, Yim AK, Liu XY, Zhou JW, Li RQ, et al. The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J Allergy Clin Immunol. 2015; 135:539–548. PMID:
25445830.

43. Thomas WR. Hierarchy and molecular properties of house dust mite allergens. Allergol Int. 2015; 64:304–311. PMID:
26433526.

44. Smith M, Cecchi L, Skjøth CA, Karrer G, Šikoparija B. Common ragweed: a threat to environmental health in Europe. Environ Int. 2013; 61:115–126. PMID:
24140540.

45. Tang R, Sun JL, Yin J, Li Z. Artemisia allergy research in China. Biomed Res Int. 2015; 2015:179426. PMID:
26000282.
46. Li J, Sun B, Huang Y, Lin X, Zhao D, Tan G, et al. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy. 2009; 64:1083–1092. PMID:
19210346.

47. Lin H, Lin R, Li N. Sensitization rates for various allergens in children with allergic rhinitis in Qingdao, China. Int J Environ Res Public Health. 2015; 12:10984–10994. PMID:
26371014.

48. Gu ZY, Li Y, Zhao CQ. Allergology ear nose throat head and neck disease. 1st ed. Beijing: People's Medical Publishing House;2012.
49. Han DM, Zhang L, Bachert C, Dong Z, Lin XP. Allergic rhinitis. 2nd ed. Beijing: People's Medical Publishing House;2014.
50. He S, Li YJ, Chen J. Clinical features of allergic rhinitis in children of Shanghai, China. Genet Mol Res. 2016; 15:1–13.

51. Wang W, Huang X, Chen Z, Zheng R, Chen Y, Zhang G, et al. Prevalence and trends of sensitisation to aeroallergens in patients with allergic rhinitis in Guangzhou, China: a 10-year retrospective study. BMJ Open. 2016; 6:e011085.

52. Simoens S, Laekeman G. Pharmacotherapy of allergic rhinitis: a pharmaco-economic approach. Allergy. 2009; 64:85–95. PMID:
19076532.

53. Chen J, Xiang J, Wang Y, Shi Q, Tan H, Kong W. Health economics analysis of specific immunotherapy in allergic rhinitis accompanied with asthma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013; 27:925–928. PMID:
24367833.
54. Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007; 28:3–9. PMID:
17390749.

55. Blaiss MS. Allergic rhinitis: direct and indirect costs. Allergy Asthma Proc. 2010; 31:375–380. PMID:
20929603.

56. Bachert C, van Cauwenberge P, Khaltaev N. World Health Organization. Allergic rhinitis and its impact on asthma. In collaboration with the World Health Organization. Executive summary of the workshop report. 7–10 December 1999, Geneva, Switzerland. Allergy. 2002; 57:841–855. PMID:
12169183.
57. Ozdoganoglu T, Songu M, Inancli HM. Quality of life in allergic rhinitis. Ther Adv Respir Dis. 2012; 6:25–39. PMID:
22032987.

58. Song Y, Wang M, Xie J, Li W, Zhang X, Wang T, et al. Prevalence of allergic rhinitis among elementary and middle school students in Changsha city and its impact on quality of life. J Laryngol Otol. 2015; 129:1108–1114. PMID:
26391176.

59. Yin Y, Lu QY. Analysis of quality of life and emotion symptoms in adolescents with allergic rhinitis in middle area of Jiangsu province. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016; 51:86–89. PMID:
26898861.
60. Ke X, Qian D, Zhu L, Hong S. Analysis on quality of life and personality characteristics of allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010; 24:200–202. PMID:
20464984.
61. Liu G, Zhu R, Wang Z, Huang A, Li W, Zhang W, et al. Assessment of quality of life in allergic rhinitis patients with Chinese version of SF-36. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005; 19:541–542. PMID:
16176005.
62. Wang Y, Zhu R, Liu G, Li W, Chen H, Daurès JP, et al. Prevalence of uncontrolled allergic rhinitis in Wuhan, China: a prospective cohort study. Am J Rhinol Allergy. 2014; 28:397–403. PMID:
25198026.

63. Huang ZZ, Zhang GH, Zhao G, Ye J, Liu X, Chen YL, et al. Clinical research on the quality of life in patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010; 45:450–454. PMID:
21055320.
64. Li L, Guan K. Quality of life in 164 allergic rhinitis patients caused by different aeroallergens. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015; 29:226–229. PMID:
26012293.
65. Xi L, Zhang Y, Han D, Zhang L. Effect of asthma, aeroallergen category, and gender on the psychological status of patients with allergic rhinitis. J Investig Allergol Clin Immunol. 2012; 22:264–269.
66. Lv X, Han D, Xi L, Zhang L. Psychological aspects of female patients with moderate-to-severe persistent allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2010; 72:235–241. PMID:
20689336.

67. Cao R, Xu Y, Tao Z, Zhang Y, Chen W, Deng A. Analysis of symptoms and quality of life in children with allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010; 24:1071–1073. 1076PMID:
21365930.
68. Sha JC, Zhu DD, Dong Z, Jiang XD, Li L, Zhu XW, et al. Survey on clinical characteristics of pediatric allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 46:26–30. PMID:
21429332.
69. Maspero J, Lee BW, Katelaris CH, Potter PC, Cingi C, Lopatin A, et al. Quality of life and control of allergic rhinitis in patients from regions beyond western Europe and the United States. Clin Exp Allergy. 2012; 42:1684–1696. PMID:
23181786.

70. Small M, Piercy J, Demoly P, Marsden H. Burden of illness and quality of life in patients being treated for seasonal allergic rhinitis: a cohort survey. Clin Transl Allergy. 2013; 3:33. PMID:
24107462.

71. Adebola SO, Abidoye B, Ologe FE, Adebola OE, Oyejola BA. Health-related quality of life and its contributory factors in allergic rhinitis patients in Nigeria. Auris Nasus Larynx. 2016; 43:171–175. PMID:
26299197.

72. Miraglia Del Giudice M, Marseglia A, Leonardi S, La Rosa M, Salpietro C, Brunese FP, et al. Allergic rhinitis and quality of life in children. Int J Immunopathol Pharmacol. 2011; 24:25–28.

73. Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008; 70:102–116. PMID:
18158379.

74. Cuffel B, Wamboldt M, Borish L, Kennedy S, Crystal-Peters J. Economic consequences of comorbid depression, anxiety, and allergic rhinitis. Psychosomatics. 1999; 40:491–496. PMID:
10581977.

75. Postolache TT, Stiller JW, Herrell R, Goldstein MA, Shreeram SS, Zebrak R, et al. Tree pollen peaks are associated with increased nonviolent suicide in women. Mol Psychiatry. 2005; 10:232–235. PMID:
15599378.

76. Sansone RA, Sansone LA. Allergic rhinitis: relationships with anxiety and mood syndromes. Innov Clin Neurosci. 2011; 8:12–17.
77. Qin P, Mortensen PB, Waltoft BL, Postolache TT. Allergy is associated with suicide completion with a possible mediating role of mood disorder - a population-based study. Allergy. 2011; 66:658–664. PMID:
21143241.

78. Lv X, Xi L, Han D, Zhang L. Evaluation of the psychological status in seasonal allergic rhinitis patients. ORL J Otorhinolaryngol Relat Spec. 2010; 72:84–90. PMID:
20431318.

79. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001; 108:S2–S8. PMID:
11449200.

80. Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Chinese Otorhinolaryngology Society of Chinese Medical Association. Diagnostic and treatment principle for allergic rhinitis and a recommended scheme. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2005; 40:166–167. PMID:
15952561.
81. Rondon C, Fernandez J, Canto G, Blanca M. Local allergic rhinitis: concept, clinical manifestations, and diagnostic approach. J Investig Allergol Clin Immunol. 2010; 20:364–371.
82. Gómez F, Rondón C, Salas M, Campo P. Local allergic rhinitis: mechanisms, diagnosis and relevance for occupational rhinitis. Curr Opin Allergy Clin Immunol. 2015; 15:111–116. PMID:
25961385.
83. Dordal MT, Lluch-Bernal M, Sánchez MC, Rondón C, Navarro A, Montoro J, et al. Allergen-specific nasal provocation testing: review by the rhinoconjunctivitis committee of the Spanish Society of Allergy and Clinical Immunology. J Investig Allergol Clin Immunol. 2011; 21:1–12.
84. Rondón C, Campo P, Herrera R, Blanca-Lopez N, Melendez L, Canto G, et al. Nasal allergen provocation test with multiple aeroallergens detects polysensitization in local allergic rhinitis. J Allergy Clin Immunol. 2011; 128:1192–1197. PMID:
21783237.

85. Rondón C, Campo P, Togias A, Fokkens WJ, Durham SR, Powe DG, et al. Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol. 2012; 129:1460–1467. PMID:
22516477.

86. Rondón C, Campo P, Zambonino MA, Blanca-Lopez N, Torres MJ, Melendez L, et al. Follow-up study in local allergic rhinitis shows a consistent entity not evolving to systemic allergic rhinitis. J Allergy Clin Immunol. 2014; 133:1026–1031. PMID:
24332860.

87. van Beijsterveldt CE, Boomsma DI. Genetics of parentally reported asthma, eczema and rhinitis in 5-yr-old twins. Eur Respir J. 2007; 29:516–521. PMID:
17215318.

88. Feijen M, Gerritsen J, Postma DS. Genetics of allergic disease. Br Med Bull. 2000; 56:894–907. PMID:
11359627.

89. Ma L, Chen DL, Zhang RX, Wang XL, Shi YJ, Ji C, et al. Genetic epidemiological study on allergic rhinitis in Nantong region of Jiangsu province. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007; 42:643–646. PMID:
18051559.
90. Haagerup A, Bjerke T, Schøitz PO, Binderup HG, Dahl R, Kruse TA. Allergic rhinitis--a total genome-scan for susceptibility genes suggests a locus on chromosome 4q24–q27. Eur J Hum Genet. 2001; 9:945–952. PMID:
11840197.
91. Haagerup A, Børglum AD, Binderup HG, Kruse TA. Fine-scale mapping of type I allergy candidate loci suggests central susceptibility genes on chromosomes 3q, 4q and Xp. Allergy. 2004; 59:88–94. PMID:
14674939.

92. Yokouchi Y, Shibasaki M, Noguchi E, Nakayama J, Ohtsuki T, Kamioka M, et al. A genome-wide linkage analysis of orchard grass-sensitive childhood seasonal allergic rhinitis in Japanese families. Genes Immun. 2002; 3:9–13. PMID:
11857054.

93. Lin S, Liu R, Guo H. Detection of antigen specificities of HLA-A, B loci in perennial allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1997; 32:18–20. PMID:
10743120.
94. Yang L, Zhang Q, Zhang P. Analysis of HLA-DRB1 allele polymorphism for patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1999; 34:147–149. PMID:
12764804.
95. Xing Z, Yu D. Linkage of allergic rhinitis with HLA-DRB alleles polymorphism. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2001; 15:199–201. PMID:
12541760.
96. Xing Z, Yu D, An S. Association of hypersensitivity to wormwood pollen in patients with allergic rhinitis with HLA alleles polymorphism. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002; 16:678–680. PMID:
12669444.
97. Wang M, Xing ZM, Yu DL, Yan Z, Yu LS. Association between HLA class II locus and the susceptibility to Artemisia pollen-induced allergic rhinitis in Chinese population. Otolaryngol Head Neck Surg. 2004; 130:192–196. PMID:
14990915.

98. Cui Z, Zhang H, Liu Y, Yang Y, Xiang Y. Analysis of HLA-DQB1 polymorphism for patients with allergic rhinitis of Uygur and Han people in Xinjiang. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 25:645–648. PMID:
22032123.
99. Zhao Y, Zhao Y, Li J, Zhang Y, Zhang L. HLA-DRB1
*08:03:02 and HLA-DQB1
*06:01:01 are associated with house dust mite-sensitive allergic rhinitis in Chinese subjects. Int Forum Allergy Rhinol. 2016; 6:854–861. PMID:
27013183.
100. Andiappan AK, Rotzschke O, Wang Y, Chew FT. Association of interleukin-13 SNP rs20541 (Arg>Gln) to allergic rhinitis in an Asian population of ethnic Chinese in Singapore. Gene. 2013; 529:357–358. PMID:
23954258.
101. Ying XJ, Zhao SW, Wang GL, Xie J, Xu HM, Dong P. Association of interleukin-13 SNP rs20541 with allergic rhinitis risk: a meta-analysis. Gene. 2013; 521:222–226. PMID:
23545317.

102. Robertson JI. Antihypertensive drug therapy: achievements, failures and prospects. Neth J Med. 1990; 37:89–93. PMID:
2250762.
103. Zhang Y, Li J, Wang C, Zhang L. Association between the interaction of key genes involved in effector T-cell pathways and susceptibility to develop allergic rhinitis: a population-based case-control association study. PLoS One. 2015; 10:e0131248. PMID:
26196693.
104. Wang M, Zhang Y, Han D, Zhang L. Association between polymorphisms in cytokine genes IL-17A and IL-17F and development of allergic rhinitis and comorbid asthma in Chinese subjects. Hum Immunol. 2012; 73:647–653. PMID:
22507625.

105. Zhao N, Liu HJ, Sun YY, Li YZ. Role of interleukin-6 polymorphisms in the development of allergic rhinitis. Genet Mol Res. 2016; 15:1–6.

106. Shen Y, Yuan XD, Hu D, Ke X, Wang XQ, Hu GH, et al. Association between interleukin-27 gene polymorphisms and susceptibility to allergic rhinitis. Hum Immunol. 2014; 75:991–995. PMID:
25075448.

107. Hu D, Hu G, Zhu J, Shen Y, Kang H, Hong S. Association between polymorphisms of the IL-23R gene and allergic rhinitis in a Chinese Han population. PLoS One. 2013; 8:e63858. PMID:
23696856.

108. Wei P, Kou W, Sun R, Hu GH, Hu D, Feng J, et al. Erratum to: association study between interleukin-12 receptor β1/β2 genes and allergic rhinitis in the Chinese Han population. Eur Arch Otorhinolaryngol. 2015; 272:895–896. PMID:
25082175.

109. Zhang Y, Duan S, Wei X, Zhao Y, Zhao L, Zhang L. Association between polymorphisms in FOXP3 and EBI3 genes and the risk for development of allergic rhinitis in Chinese subjects. Hum Immunol. 2012; 73:939–945. PMID:
22836044.

110. Shen Y, Liu Y, Ke X, Kang HY, Hu GH, Hong SL. Association between JAK1 gene polymorphisms and susceptibility to allergic rhinitis. Asian Pac J Allergy Immunol. 2016; 34:124–129. PMID:
27007833.

111. Gu Z, Hong SL, Ke X, Shen Y, Wang XQ, Hu D, et al. FCRL3 gene polymorphisms confer autoimmunity risk for allergic rhinitis in a Chinese Han population. PLoS One. 2015; 10:e0116419. PMID:
25594855.

112. Liu Y, Ke X, Kang HY, Wang XQ, Shen Y, Hong SL. Genetic risk of TNFSF4 and FAM167A-BLK polymorphisms in children with asthma and allergic rhinitis in a Han Chinese population. J Asthma. 2016; 53:567–575. PMID:
27088737.

113. Yang KD, Liu CA, Chang JC, Chuang H, Ou CY, Hsu TY, et al. Polymorphism of the immune-braking gene CTLA-4 (+49) involved in gender discrepancy of serum total IgE levels and allergic diseases. Clin Exp Allergy. 2004; 34:32–37. PMID:
14720259.

114. Han D, She W, Zhang L. Association of the CD14 gene polymorphism C-159T with allergic rhinitis. Am J Rhinol Allergy. 2010; 24:e1–e3. PMID:
20109306.

115. Wei X, Zhang Y, Fu Z, Zhang L. The association between polymorphisms in the MRPL4 and TNF-α genes and susceptibility to allergic rhinitis. PLoS One. 2013; 8:e57981. PMID:
23472126.

116. Zhao Y, Zhang Y, Zhang L. Variant of PBX2 gene in the 6p21.3 asthma susceptibility locus is associated with allergic rhinitis in Chinese subjects. Int Forum Allergy Rhinol. 2016; 6:537–543. PMID:
26852910.

117. Chen Q, Liu Z, Zhang H. Relationship between rs1057141 and rs1135216 polymorphisms of TAP1 gene and allergic rhinitis in Xinjiang Han people. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012; 26:917–920. 925PMID:
23272491.
118. Ke X, Yang Y, Shen Y, Wang X, Hong S. Association between TNFAIP3 gene polymorphisms and risk of allergic rhinitis in a Chinese Han population. Iran J Allergy Asthma Immunol. 2016; 15:46–52. PMID:
26996111.
119. Wang XD, Zhang L, Duan H, She WY, Zhao Y, Liu S, et al. Association of single nucleotide polymorphisms of GATA3 with allergic rhinitis phenotypes in Chinese. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008; 43:494–498. PMID:
18826115.
120. Zhang Y, Lin X, Desrosiers M, Zhang W, Meng N, Zhao L, et al. Association pattern of interleukin-1 receptor-associated kinase-4 gene polymorphisms with allergic rhinitis in a Han Chinese population. PLoS One. 2011; 6:e21769. PMID:
21738793.

121. Zhu XJ, Zhu LP, Lu MP, Wang ML, Qi QH, Yin M, et al. Association of TGFB1 gene polymorphism -509C/T with disease severity in childhood allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010; 45:459–464. PMID:
21055322.
122. Jin P, Andiappan AK, Quek JM, Lee B, Au B, Sio YY, et al. A functional brain-derived neurotrophic factor (BDNF) gene variant increases the risk of moderate-to-severe allergic rhinitis. J Allergy Clin Immunol. 2015; 135:1486–1493.e8. PMID:
25649076.

123. Tian HQ, Chen XY, Lu Y, Lu WM, Wang ML, Zhao HL, et al. Association of VDR and CYP2R1 polymorphisms with mite-sensitized persistent allergic rhinitis in a Chinese population. PLoS One. 2015; 10:e0133162. PMID:
26177022.

124. Huang RF, Dong P, Zhang TZ, Ying XJ, Hu H. Angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to allergic rhinitis in Chinese populations: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2016; 273:277–283. PMID:
25341696.

125. Guo M, Ma J, Han Y, Lu L. Angiotensin-converting enzyme gene insertion/deletion polymorphisms and the susceptibility to allergic rhinitis. Allergol Immunopathol (Madr). 2014; 42:568–572. PMID:
24637107.

126. Lin H, Lin D, Zheng CQ. Angiotensin-converting enzyme insertion/deletion polymorphism associated with allergic rhinitis susceptibility: evidence from 1,410 subjects. J Renin Angiotensin Aldosterone Syst. 2014; 15:593–600. PMID:
24150611.

127. Li Z, Yan F, Yang Z, Zhou J, Chen Y, Ding Z. Association between ADAM33 S2 and V4 polymorphisms and susceptibility to allergic rhinitis: a meta-analysis. Allergol Immunopathol (Madr). 2016; 44:170–176. PMID:
26619918.

128. Chen RX, Lu WM, Zhu LP, Lu MP, Wang ML, Wang YL, et al. Association study on ADAM33 polymorphisms in mite-sensitized persistent allergic rhinitis in a Chinese population. PLoS One. 2014; 9:e95033. PMID:
24751681.

129. Tang XF, Tang HY, Sun LD, Xiao FL, Zhang Z, Li Y, et al. Genetic variant rs4982958 at 14q11.2 is associated with allergic rhinitis in a Chinese Han population running title: 14q11.2 is a susceptibility locus for allergic rhinitis. J Investig Allergol Clin Immunol. 2012; 22:55–62.
130. Lu MP, Chen RX, Wang ML, Zhu XJ, Zhu LP, Yin M, et al. Association study on IL4, IL13 and IL4RA polymorphisms in mite-sensitized persistent allergic rhinitis in a Chinese population. PLoS One. 2011; 6:e27363. PMID:
22087298.

131. Li JY, Zhang Y, Lin XP, Ruan Y, Wang Y, Wang CS, et al. Association between DNA hypomethylation at IL13 gene and allergic rhinitis in house dust mite-sensitized subjects. Clin Exp Allergy. 2016; 46:298–307. PMID:
26399722.

132. Andiappan AK, Wang DY, Anantharaman R, Parate PN, Suri BK, Low HQ, et al. Genome-wide association study for atopy and allergic rhinitis in a Singapore Chinese population. PLoS One. 2011; 6:e19719. PMID:
21625490.

133. Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008; 9:839–845. PMID:
18645592.

134. Teng Y, Zhang R, Yu H, Wang H, Hong Z, Zhuang W, et al. Altered MicroRNA expression profiles in activated mast cells following IgE-FcεRI cross-linking with antigen. Cell Physiol Biochem. 2015; 35:2098–2110. PMID:
25895812.

135. Shaoqing Y, Ruxin Z, Guojun L, Zhiqiang Y, Hua H, Shudong Y, et al. Microarray analysis of differentially expressed microRNAs in allergic rhinitis. Am J Rhinol Allergy. 2011; 25:e242–e246. PMID:
22185732.

136. Teng Y, Zhang R, Liu C, Zhou L, Wang H, Zhuang W, et al. miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Rα1. Biochem Biophys Res Commun. 2015; 457:58–64. PMID:
25529447.

137. Luo Y, Deng Y, Tao Z, Chen S, Xiao B, Ren J, et al. Regulatory effect of microRNA-135a on the Th1/Th2 imbalance in a murine model of allergic rhinitis. Exp Ther Med. 2014; 8:1105–1110. PMID:
25187805.

138. Suojalehto H, Toskala E, Kilpeläinen M, Majuri ML, Mitts C, Lindström I, et al. MicroRNA profiles in nasal mucosa of patients with allergic and nonallergic rhinitis and asthma. Int Forum Allergy Rhinol. 2013; 3:612–620. PMID:
23704072.

139. Chen RF, Huang HC, Ou CY, Hsu TY, Chuang H, Chang JC, et al. MicroRNA-21 expression in neonatal blood associated with antenatal immunoglobulin E production and development of allergic rhinitis. Clin Exp Allergy. 2010; 40:1482–1490. PMID:
20701609.

140. Hansen I, Klimek L, Mösges R, Hörmann K. Mediators of inflammation in the early and the late phase of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2004; 4:159–163. PMID:
15126935.

141. Dullaers M, De Bruyne R, Ramadani F, Gould HJ, Gevaert P, Lambrecht BN. The who, where, and when of IgE in allergic airway disease. J Allergy Clin Immunol. 2012; 129:635–645. PMID:
22168998.

142. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454. PMID:
18650915.

143. Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012; 18:684–692. PMID:
22561832.

144. Kamekura R, Yamashita K, Jitsukawa S, Nagaya T, Ito F, Ichimiya S, et al. Role of crosstalk between epithelial and immune cells, the epimmunome, in allergic rhinitis pathogenesis. Adv Otorhinolaryngol. 2016; 77:75–82. PMID:
27116609.
145. Takizawa R, Pawankar R, Yamagishi S, Takenaka H, Yagi T. Increased expression of HLA-DR and CD86 in nasal epithelial cells in allergic rhinitics: antigen presentation to T cells and up-regulation by diesel exhaust particles. Clin Exp Allergy. 2007; 37:420–433. PMID:
17359392.

146. Fukuoka A, Matsushita K, Morikawa T, Takano H, Yoshimoto T. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin Exp Allergy. 2016; 46:142–152. PMID:
26201369.

147. Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001; 2:816–822. PMID:
11526392.

148. Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009; 123:1004–1011. PMID:
19410689.

149. Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014; 133:1203–1205.e7. PMID:
24582313.

150. Panganiban RP, Wang Y, Howrylak J, Chinchilli VM, Craig TJ, August A, et al. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016; 137:1423–1432. PMID:
27025347.

151. Kim AS, Doherty TA, Karta MR, Das S, Baum R, Rosenthal P, et al. Regulatory B cells and T follicular helper cells are reduced in allergic rhinitis. J Allergy Clin Immunol. 2016; 138:1192–1195.e5. PMID:
27142393.

152. He S, Zhang H, Zeng X, Yang P. Self-amplification mechanisms of mast cell activation: a new look in allergy. Curr Mol Med. 2012; 12:1329–1339. PMID:
22920722.

153. Law M, Morales JL, Mottram LF, Iyer A, Peterson BR, August A. Structural requirements for the inhibition of calcium mobilization and mast cell activation by the pyrazole derivative BTP2. Int J Biochem Cell Biol. 2011; 43:1228–1239. PMID:
21558014.

154. Spiegl N, Didichenko S, McCaffery P, Langen H, Dahinden CA. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood. 2008; 112:3762–3771. PMID:
18495959.

155. Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989; 339:150–152. PMID:
2524008.

156. Zhang H, Yang H, Zhang L, Yang X, Zhang Z, Lin Q, et al. Induction of IL-4 release and upregulated expression of protease activated receptors by GM-CSF in P815 cells. Cytokine. 2009; 48:196–202. PMID:
19651524.

157. Baraniuk JN. Pathogenesis of allergic rhinitis. J Allergy Clin Immunol. 1997; 99:S763–S772. PMID:
9042069.

158. Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003; 111:24–32. PMID:
12532090.
159. Lorentz A, Wilke M, Sellge G, Worthmann H, Klempnauer J, Manns MP, et al. IL-4-induced priming of human intestinal mast cells for enhanced survival and Th2 cytokine generation is reversible and associated with increased activity of ERK1/2 and c-Fos. J Immunol. 2005; 174:6751–6756. PMID:
15905515.

160. Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002; 346:1699–1705. PMID:
12037149.

161. Järvikallio A, Naukkarinen A, Harvima IT, Aalto ML, Horsmanheimo M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol. 1997; 136:871–877. PMID:
9217819.

162. Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003; 305:1212–1221. PMID:
12626656.
163. Nilsson G, Metcalfe DD, Taub DD. Demonstration that platelet-activating factor is capable of activating mast cells and inducing a chemotactic response. Immunology. 2000; 99:314–319. PMID:
10692052.

164. Hart PH. Regulation of the inflammatory response in asthma by mast cell products. Immunol Cell Biol. 2001; 79:149–153. PMID:
11264709.

165. Misiak-Tłoczek A, Brzezińska-Błaszczyk E. IL-6, but not IL-4, stimulates chemokinesis and TNF stimulates chemotaxis of tissue mast cells: involvement of both mitogen-activated protein kinases and phosphatidylinositol 3-kinase signalling pathways. APMIS. 2009; 117:558–567. PMID:
19664126.
166. Brzezińska-Błaszczyk E, Pietrzak A, Misiak-Tłoczek AH. Tumor necrosis factor (TNF) is a potent rat mast cell chemoattractant. J Interferon Cytokine Res. 2007; 27:911–919. PMID:
18052723.

167. Toru H, Kinashi T, Ra C, Nonoyama S, Yata J, Nakahata T. Interleukin-4 induces homotypic aggregation of human mast cells by promoting LFA-1/ICAM-1 adhesion molecules. Blood. 1997; 89:3296–3302. PMID:
9129035.

168. Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008; 112:1269–1279. PMID:
18524989.

169. He S, Zhang H, Chen H, Yang H, Huang T, Chen Y, et al. Expression and release of IL-29 by mast cells and modulation of mast cell behavior by IL-29. Allergy. 2010; 65:1234–1241. PMID:
20337614.

170. Olsson N, Taub DD, Nilsson G. Regulation of mast cell migration by T and T cytokines: identification of tumour necrosis factor-alpha and interleukin-4 as mast cell chemotaxins. Scand J Immunol. 2004; 59:267–272. PMID:
15030577.
171. Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001; 97:2045–2052. PMID:
11264170.

172. Kempuraj D, Saito H, Kaneko A, Fukagawa K, Nakayama M, Toru H, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999; 93:3338–3346. PMID:
10233886.

173. Andersen HB, Holm M, Hetland TE, Dahl C, Junker S, Schiøtz PO, et al. Comparison of short term
in vitro cultured human mast cells from different progenitors - Peripheral blood-derived progenitors generate highly mature and functional mast cells. J Immunol Methods. 2008; 336:166–174. PMID:
18538784.
174. Yamaguchi M, Azuma H, Fujihara M, Hamada H, Ikeda H. Generation of a considerable number of functional mast cells with a high basal level of FcepsilonRI expression from cord blood CD34+ cells by co-culturing them with bone marrow stromal cell line under serum-free conditions. Scand J Immunol. 2007; 65:581–588. PMID:
17523952.
175. Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res. 2004; 24:271–281. PMID:
15153310.

176. Wisniewski JA, Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 2011; 32:83–94. PMID:
21439160.

177. Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010; 184:3526–3534. PMID:
20190140.
178. He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate
in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997; 159:6216–6225. PMID:
9550425.
179. He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells
in vivo. Br J Pharmacol. 1998; 125:1491–1500. PMID:
9884078.
180. Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components
in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997; 17:519–528. PMID:
9376127.
181. Dias-Baruffi M, Pereira-da-Silva G, Jamur MC, Roque-Barreira MC. Heparin potentiates
in vivo neutrophil migration induced by IL-8. Glycoconj J. 1998; 15:523–526. PMID:
9881755.
182. Thomas PS. Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunol Cell Biol. 2001; 79:132–140. PMID:
11264706.
183. Zhang H, Kong H, Zeng X, Guo L, Sun X, He S. Subsets of regulatory T cells and their roles in allergy. J Transl Med. 2014; 12:125. PMID:
24886492.

184. Jutel M, Akdis M, Blaser K, Akdis CA. Are regulatory T cells the target of venom immunotherapy? Curr Opin Allergy Clin Immunol. 2005; 5:365–369. PMID:
15985821.

185. Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008; 28:870–880. PMID:
18513999.

186. Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010; 3:216–229. PMID:
20164832.

187. Wu K, Bi Y, Sun K, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol. 2007; 4:269–275. PMID:
17764617.
188. Noble A, Giorgini A, Leggat JA. Cytokine-induced IL-10-secreting CD8 T cells represent a phenotypically distinct suppressor T-cell lineage. Blood. 2006; 107:4475–4483. PMID:
16467201.

189. Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009; 106:4793–4798. PMID:
19273860.

190. Xystrakis E, Boswell SE, Hawrylowicz CM. T regulatory cells and the control of allergic disease. Expert Opin Biol Ther. 2006; 6:121–133. PMID:
16436038.

191. Ziora D, Sitek P, Machura E, Ziora K. Bronchial asthma in obesity--a distinct phenotype of asthma? Pneumonol Alergol Pol. 2012; 80:454–462. PMID:
22926907.
192. Li L, Boussiotis VA. Control and regulation of peripheral tolerance in allergic inflammatory disease: therapeutic consequences. Chem Immunol Allergy. 2008; 94:178–188. PMID:
18802347.

193. Nieminen K, Laaksonen K, Savolainen J. Three-year follow-up study of allergen-induced
in vitro cytokine and signalling lymphocytic activation molecule mRNA responses in peripheral blood mononuclear cells of allergic rhinitis patients undergoing specific immunotherapy. Int Arch Allergy Immunol. 2009; 150:370–376. PMID:
19571569.
194. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010; 464:1367–1370. PMID:
20200518.

195. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010; 463:540–544. PMID:
20023630.

196. Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010; 107:11489–11494. PMID:
20534524.

197. Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010; 464:1362–1366. PMID:
20200520.

198. Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage(−)Sca1+c-Kit(−)CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol. 2011; 187:5795–5804. PMID:
22048767.

199. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012; 188:1503–1513. PMID:
22198948.
200. Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013; 132:933–941. PMID:
23810766.

201. Salmond RJ, Mirchandani AS, Besnard AG, Bain CC, Thomson NC, Liew FY. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012; 130:1159–1166.e6. PMID:
22738676.

202. Hung LY, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci USA. 2013; 110:282–287. PMID:
23248269.

203. Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci USA. 2012; 109:3451–3456. PMID:
22331917.

204. Lefrançais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA. 2014; 111:15502–15507. PMID:
25313073.
205. Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013; 14:536–542. PMID:
23685824.

206. Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011; 12:631–638. PMID:
21623379.

207. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012; 36:451–463. PMID:
22425247.

208. Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014; 163:92–105. PMID:
24296722.
209. Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012; 42:1106–1116. PMID:
22539286.
210. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013; 210:2939–2950. PMID:
24323357.

211. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013; 5:170ra16.

212. Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015; 136:792–794.e3. PMID:
26233928.

213. Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014; 69:1154–1161. PMID:
24924975.

214. Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015; 45:394–403. PMID:
25429730.

215. Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT Jr, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015; 136:59–68.e14. PMID:
25617223.

216. Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations
in vivo. Am J Respir Crit Care Med. 2014; 190:1373–1382. PMID:
25350863.
217. Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014; 134:1193–1195.e4. PMID:
25212194.

218. Fan D, Wang X, Wang M, Wang Y, Zhang L, Li Y, et al. Allergen-dependent differences in ILC2s frequencies in patients with allergic rhinitis. Allergy Asthma Immunol Res. 2016; 8:216–222. PMID:
26922931.

219. Zhong H, Fan XL, Yu QN, Qin ZL, Chen D, Xu R, et al. Increased innate type 2 immune response in house dust mite-allergic patients with allergic rhinitis. Clin Immunol. 2017; 183:293–299. PMID:
28917723.

220. Yu QN, Guo YB, Li X, Li CL, Tan WP, Fan XL, et al. ILC2 frequency and activity are inhibited by glucocorticoid treatment via STAT pathway in patients with asthma. Allergy. 2018; 3. 15. DOI:
10.1111/all.13438.

221. Salib RJ, Lau LC, Howarth PH. Nasal lavage fluid concentrations of eotaxin-1 (CCL11) in naturally occurring allergic rhinitis: relationship to disease activity, nasal luminal eosinophil influx, and plasma protein exudation. Clin Exp Allergy. 2005; 35:995–1002. PMID:
16120080.

222. Yan Z, Zhang R, Yu S, Wu G. Study on the expression of Eotaxin and the role of histamine in allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009; 23:1086–1088. PMID:
20359111.
223. Canonica GW, Compalati E. Minimal persistent inflammation in allergic rhinitis: implications for current treatment strategies. Clin Exp Immunol. 2009; 158:260–271. PMID:
19765020.

224. KleinJan A, Dijkstra MD, Boks SS, Severijnen LA, Mulder PG, Fokkens WJ. Increase in IL-8, IL-10, IL-13, and RANTES mRNA levels (in situ hybridization) in the nasal mucosa after nasal allergen provocation. J Allergy Clin Immunol. 1999; 103:441–450. PMID:
10069878.

225. Kaplan AP. Chemokines, chemokine receptors and allergy. Int Arch Allergy Immunol. 2001; 124:423–431. PMID:
11340325.

226. Bochner BS, Bickel CA, Taylor ML, MacGlashan DW Jr, Gray PW, Raport CJ, et al. Macrophage-derived chemokine induces human eosinophil chemotaxis in a CC chemokine receptor 3- and CC chemokine receptor 4-independent manner. J Allergy Clin Immunol. 1999; 103:527–532. PMID:
10069890.

227. Zhang RX, Yu SQ, Jiang JZ, Liu GJ. Complementary DNA microarray analysis of chemokines and their receptors in allergic rhinitis. J Investig Allergol Clin Immunol. 2007; 17:329–336.
228. Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993; 260:1946–1950. PMID:
8100368.

229. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987; 327:524–526. PMID:
3495737.

230. Lundberg JO, Rinder J, Weitzberg E, Lundberg JM, Alving K. Nasally exhaled nitric oxide in humans originates mainly in the paranasal sinuses. Acta Physiol Scand. 1994; 152:431–432. PMID:
7701944.
231. Hanazawa T, Antuni JD, Kharitonov SA, Barnes PJ. Intranasal administration of eotaxin increases nasal eosinophils and nitric oxide in patients with allergic rhinitis. J Allergy Clin Immunol. 2000; 105:58–64. PMID:
10629453.

232. Yu S, Yan Z, Che N, Zhang X, Ge R. Impact of carbon monoxide/heme oxygenase on hydrogen sulfide/cystathionine-γ-lyase pathway in the pathogenesis of allergic rhinitis in guinea pigs. Otolaryngol Head Neck Surg. 2015; 152:470–476. PMID:
25583855.

233. Shaoqing Y, Ruxin Z, Yingjian C, Jianqiu C, Yanshen W, Genhong L. A meta-analysis of the association of exhaled carbon monoxide on asthma and allergic rhinitis. Clin Rev Allergy Immunol. 2011; 41:67–75. PMID:
20094823.

234. Park SJ, Kim TH, Lee SH, Ryu HY, Hong KH, Jung JY, et al. Expression levels of endogenous hydrogen sulfide are altered in patients with allergic rhinitis. Laryngoscope. 2013; 123:557–563. PMID:
23303708.

235. Shaoqing Y, Ruxin Z, Yinjian C, Jianqiu C, Zhiqiang Y, Genhong L. Down-regulation of endogenous hydrogen sulphide pathway in nasal mucosa of allergic rhinitis in guinea pigs. Allergol Immunopathol (Madr). 2009; 37:180–187. PMID:
19783349.

236. Lundberg JM, Brodin E, Hua X, Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand. 1984; 120:217–227. PMID:
6201040.

237. Baraniuk JN, Lundgren JD, Okayama M, Goff J, Mullol J, Merida M, et al. Substance P and neurokinin A in human nasal mucosa. Am J Respir Cell Mol Biol. 1991; 4:228–236. PMID:
1705809.

238. Zhang R, Jiang D, Li Z. Experimental study on blocking agent of substance P nerves in the treatment of allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1994; 29:282–285. PMID:
7536431.
239. Zhang R, Jiang D, Li Z. Clinical observation and therapeutic mechanism of blocking agent of substance P nerves in the treatment of perennial allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1995; 30:163–165. PMID:
8579875.
240. Hu H, Zhang R, Fang X, Yu M, Yu S, Zhang J, et al. Effects of endogenous substance P expression on degranulation in RBL-2H3 cells. Inflamm Res. 2011; 60:541–546. PMID:
21190122.

241. Hanf G, Schierhorn K, Brunnée T, Noga O, Verges D, Kunkel G. Substance P induced histamine release from nasal mucosa of subjects with and without allergic rhinitis. Inflamm Res. 2000; 49:520–523. PMID:
11089903.

242. Wang H, Zhang R, Wu J, Hu H. Knockdown of neurokinin-1 receptor expression by small interfering RNA prevents the development of allergic rhinitis in rats. Inflamm Res. 2013; 62:903–910. PMID:
23934070.

243. Liu ZG, Song JJ, Kong XL. A study on pollen allergens in China. Biomed Environ Sci. 2010; 23:319–322. PMID:
20934121.

244. Qiao BS. Airborne pollens and plants in China. Beijing: Chinese Peking Union Medical Publishing House;2005.
245. Liu GH, Zhu RF, Zhang W, Li WJ, Wang ZX, Chen H. Survey of airborne pollen in Hubei province of China. Chin Med Sci J. 2008; 23:212–217. PMID:
19180881.

246. Ouyang YH, Zhang DS, Fan EZ, Li Y, Zhang L. Correlation between symptoms of pollen allergic rhinitis and pollen grain spreading in summer and autumn. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012; 47:623–627. PMID:
23141390.
247. Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol. 2005; 115:689–699. PMID:
15805986.

248. Zhang F, Wang W, Lv J, Krafft T, Xu J. Time-series studies on air pollution and daily outpatient visits for allergic rhinitis in Beijing, China. Sci Total Environ. 2011; 409:2486–2492. PMID:
21514624.

249. Hwang BF, Jaakkola JJ, Lee YL, Lin YC, Guo YL. Relation between air pollution and allergic rhinitis in Taiwanese schoolchildren. Respir Res. 2006; 7:23. PMID:
16469096.

250. Diaz-Sanchez D, Tsien A, Casillas A, Dotson AR, Saxon A. Enhanced nasal cytokine production in human beings after
in vivo challenge with diesel exhaust particles. J Allergy Clin Immunol. 1996; 98:114–123. PMID:
8765825.
251. Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human
in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997; 158:2406–2413. PMID:
9036991.
252. Fujieda S, Diaz-Sanchez D, Saxon A. Combined nasal challenge with diesel exhaust particles and allergen induces
in vivo IgE isotype switching. Am J Respir Cell Mol Biol. 1998; 19:507–512. PMID:
9730879.
253. Diaz-Sanchez D, Penichet-Garcia M, Saxon A. Diesel exhaust particles directly induce activated mast cells to degranulate and increase histamine levels and symptom severity. J Allergy Clin Immunol. 2000; 106:1140–1146. PMID:
11112898.

254. Knox RB, Suphioglu C, Taylor P, Desai R, Watson HC, Peng JL, et al. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: implications for asthma and air pollution. Clin Exp Allergy. 1997; 27:246–251. PMID:
9088650.

255. Pandya RJ, Solomon G, Kinner A, Balmes JR. Diesel exhaust and asthma: hypotheses and molecular mechanisms of action. Environ Health Perspect. 2002; 110(Suppl 1):103–112.

256. Han YY, Forno E, Gogna M, Celedón JC. Obesity and rhinitis in a nationwide study of children and adults in the United States. J Allergy Clin Immunol. 2016; 137:1460–1465. PMID:
26883461.

257. Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Camargo CA Jr, et al. Prenatal, perinatal, and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J Allergy Clin Immunol. 2016; 137:1063–1070.e2. PMID:
26874366.

258. Kawamoto Y, Ueno Y, Nakahashi E, Obayashi M, Sugihara K, Qiao S, et al. Prevention of allergic rhinitis by ginger and the molecular basis of immunosuppression by 6-gingerol through T cell inactivation. J Nutr Biochem. 2016; 27:112–122. PMID:
26403321.

259. Nwaru BI, Takkinen HM, Kaila M, Erkkola M, Ahonen S, Pekkanen J, et al. Food diversity in infancy and the risk of childhood asthma and allergies. J Allergy Clin Immunol. 2014; 133:1084–1091. PMID:
24472626.

260. Gong W, Feng Y, Yan P, Li S, Yu C, Zhou X, et al. Effect of nasal instillation of vitamin D3 on patient with allergic rhinitis symptoms. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014; 28:1031–1033. PMID:
25330636.
261. Sabounchi S, Bollyky J, Nadeau K. Review of environmental impact on the epigenetic regulation of atopic diseases. Curr Allergy Asthma Rep. 2015; 15:33. PMID:
26141578.

262. Miller DR, Turner SW, Spiteri-Cornish D, Scaife AR, Danielian PJ, Devereux GS, et al. Maternal vitamin D and E intakes during early pregnancy are associated with airway epithelial cell responses in neonates. Clin Exp Allergy. 2015; 45:920–927. PMID:
25616026.

263. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010; 107:11971–11975. PMID:
20566857.

264. Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015; 17:592–602. PMID:
25974301.

265. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016; 352:539–544. PMID:
27126036.
266. The State Administration of Traditional Chinese medicine. Standards for diagnosis and curative effect of Chinese medical symptom. Nanjing University Press;2001.
267. Shizhen W. Chinese otorhinolaryngology. Beijing: China Traditional Chinese Medicine Publishing House;2003.
268. Guruijin . Several important issues of ear nasal allergy and immunity. Chinese J Otorhinolaryngol. 1992; 27:3011.
269. Wen Z, Tao ZD, Cao GZ. Cyclic nucleotide. Ion and the autonomic nervous system functions of patients with perennial allergic rhinitis. J Hunan Med Univ. 1990; 17:551.
270. Shen ZY. Research on warming yang herbs to prevent seasonal asthmatic attack and its principles. J Integr Tradit West Med. 1986; 6:L1.
271. Zhao JY, Liu ZB, Wu HQ. Observation of T-lymphocyte subsets in patients with lung qi deficiency and lung-yin deficiency. J Anhui Coll Tradit Chinese Med. 1993; 12:49.
272. The Wenzhou Medical Sciences. Preliminary report on the relationship between chronic bronchitis of plasma cyclic nucleotide levels and TCM differentiation of Zangfu. Zhejiang J Tradit Chinese Med. 1981; 16:2.
273. Lin WS, Xiong ZM, Zhang ZK. Relationship between nasal secretion of cyclic nucleotide levels and syndrome differentiation of chronic bronchitis. Zhejiang J Tradit Chinese Med. 1982; 17:5221.
274. Lu DW, Wang MH. Correlation research on allergic rhinitis based on syndrome differentiation of traditional Chinese and allergy index. J Integr Chinese West Med Ear Nose Throat. 1996; 4:1121.
275. Liao YH, Li YY, Chen H. Proven case of Zuwang Gan by using metal-clearing method for allergic rhinitis. J Guangzhou Univ Tradit Chinese Med. 2004; 21:154–156.
276. Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014; 69:854–867. PMID:
24761804.

277. Demoly P, Michel F, Bousquet J. In vivo methods for study of allergy: skin tests, techniques and interpretation. In : Middleton E, Reed CE, Ellis EF, Adkinson NF, Yunginger JW, Busse WW, editors. Allergy: principles and practice. 5th ed. St Louis (MO): Mosby Co;1998.
278. Sub-Committee on Skin Tests of the European Academy of Allergology and Clinical Immunology. Skin tests used in type I allergy testing Position paper. Allergy. 1989; 44(Suppl 10):1–59.
279. Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities from immunotherapy (IT) and skin testing (ST). J Allergy Clin Immunol. 1987; 79:660–677. PMID:
3559001.

280. Reid MJ, Lockey RF, Turkeltaub PC, Platts-Mills TA. Survey of fatalities from skin testing and immunotherapy 1985–1989. J Allergy Clin Immunol. 1993; 92:6–15. PMID:
8335856.

281. The European Academy of Allergology and Clinical Immunology. Position paper: allergen standardization and skin tests. Allergy. 1993; 48(Suppl):48–82. PMID:
8342740.
282. Allergen immunotherapy: therapeutic vaccines for allergic diseases. Geneva: January 27–29 1997. Allergy. 1998; 53(44 Suppl):1–42.
283. Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic-rhinitis patients with negative skin tests. Lancet. 1975; 2:148–150. PMID:
49744.

284. Board of Directors. American Academy of Allergy and Immunology. Allergen skin testing. J Allergy Clin Immunol. 1993; 92:636–637. PMID:
8227853.
285. Miadonna A, Leggieri E, Tedeschi A, Zanussi C. Clinical significance of specific IgE determination on nasal secretion. Clin Allergy. 1983; 13:155–164. PMID:
6839442.

286. Deuschl H, Johansson SG. Specific IgE antibodies in nasal secretion from patients with allergic rhinitis and with negative or weakly positive RAST on the serum. Clin Allergy. 1977; 7:195–202. PMID:
872365.

287. Osterballe O, Weeke B. A new lancet for skin prick testing. Allergy. 1979; 34:209–212. PMID:
391093.

288. Adinoff AD, Rosloniec DM, McCall LL, Nelson HS. Immediate skin test reactivity to Food and Drug Administration-approved standardized extracts. J Allergy Clin Immunol. 1990; 86:766–774. PMID:
2229841.

289. Simons FE, Johnston L, Gu X, Simons KJ. Suppression of the early and late cutaneous allergic responses using fexofenadine and montelukast. Ann Allergy Asthma Immunol. 2001; 86:44–50. PMID:
11206237.

290. Hill SL 3rd, Krouse JH. The effects of montelukast on intradermal wheal and flare. Otolaryngol Head Neck Surg. 2003; 129:199–203. PMID:
12958567.

291. Wide L, Bennich H, Johansson SG. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967; 2:1105–1107. PMID:
4168552.

292. Johansson SG, Bennich H, Foucard T. Quantitation of IgE antibodies and allergens by the radioallergosorbent test, RAST. Int Arch Allergy Appl Immunol. 1973; 45:55–56. PMID:
4580379.

293. Bousquet J, Chanez P, Chanal I, Michel FB. Comparison between RAST and Pharmacia CAP system: a new automated specific IgE assay. J Allergy Clin Immunol. 1990; 85:1039–1043. PMID:
2191989.

294. Pastorello EA, Incorvaia C, Pravettoni V, Marelli A, Farioli L, Ghezzi M. Clinical evaluation of CAP System and RAST in the measurement of specific IgE. Allergy. 1992; 47:463–466. PMID:
1485648.

295. Chinoy B, Yee E, Bahna SL. Skin testing versus radioallergosorbent testing for indoor allergens. Clin Mol Allergy. 2005; 3:4. PMID:
15833110.

296. Eriksson NE. Allergy screening with Phadiatop and CAP Phadiatop in combination with a questionnaire in adults with asthma and rhinitis. Allergy. 1990; 45:285–292. PMID:
2382793.

297. Kam KL, Hsieh KH. Comparison of three
in vitro assays for serum IgE with skin testing in asthmatic children. Ann Allergy. 1994; 73:329–336. PMID:
7944001.
298. Lloyd GA, Lund VJ, Scadding GK. CT of the paranasal sinuses and functional endoscopic surgery: a critical analysis of 100 symptomatic patients. J Laryngol Otol. 1991; 105:181–185. PMID:
2019802.

299. Mafee MF, Chow JM, Meyers R. Functional endoscopic sinus surgery: anatomy, CT screening, indications, and complications. AJR Am J Roentgenol. 1993; 160:735–744. PMID:
8456654.

300. Leipzig JR, Martin DS, Eisenbeis JF, Slavin RG. Computed tomographic study of the paranasal sinuses in allergic rhinitis. J Allergy Clin Immunol. 1996; 98:1130–1131. PMID:
8977520.

301. Bhattacharyya N, Fried MP. The accuracy of computed tomography in the diagnosis of chronic rhinosinusitis. Laryngoscope. 2003; 113:125–129. PMID:
12514395.

302. Mafee MF, Tran BH, Chapa AR. Imaging of rhinosinusitis and its complications: plain film, CT, and MRI. Clin Rev Allergy Immunol. 2006; 30:165–186. PMID:
16785588.

303. Galassi C, De Sario M, Biggeri A, Bisanti L, Chellini E, Ciccone G, et al. Changes in prevalence of asthma and allergies among children and adolescents in Italy: 1994–2002. Pediatrics. 2006; 117:34–42. PMID:
16396858.

304. Hytönen M, Sala E. Nasal provocation test in the diagnostics of occupational allergic rhinitis. Rhinology. 1996; 34:86–90. PMID:
8876069.
305. Eggleston PA, Ansari AA, Adkinson NF Jr, Wood RA. Environmental challenge studies in laboratory animal allergy. Effect of different airborne allergen concentrations. Am J Respir Crit Care Med. 1995; 151:640–646. PMID:
7881650.

306. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930. PMID:
15817806.
307. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615. PMID:
21885636.

308. Struben VM, Wieringa MH, Feenstra L, de Jongste JC. Nasal nitric oxide and nasal allergy. Allergy. 2006; 61:665–670. PMID:
16677234.

309. Chen W, Purohit A, Barnig C, Casset A, de Blay F. Niox and Niox Mino: comparison of exhaled NO in grass pollen allergic adult volunteers. Allergy. 2007; 62:571–572. PMID:
17441801.
310. Olin AC, Alving K, Torén K. Exhaled nitric oxide: relation to sensitization and respiratory symptoms. Clin Exp Allergy. 2004; 34:221–226. PMID:
14987301.

311. Zhang L, Luo XR, Liu CY, Zhao Y, Han DM. Measurement of exhaled nitric oxide in healthy Chinese. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009; 44:302–306. PMID:
19558836.
312. Leng G, Li Z, Wang Q. Detection of exhaled nitric oxide of healthy in Nanjing. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012; 26:769–771.
313. Liu D, Huang Z, Huang Y, Yi X, Chen X. Measurement of nasal and fractional exhaled nitric oxide in children with upper airway inflammatory disease: preliminary results. Int J Pediatr Otorhinolaryngol. 2015; 79:2308–2311. PMID:
26602553.

314. You S, Zhang J, Ji L, Bai Y, Wang H. Noninvasive measurement of nasal NO and fractional exhaled NO in healthy people and patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014; 49:323–325. PMID:
24931022.
315. Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Suggestion on the diagnosis and treatment of vasomotor rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013; 48:884–885. PMID:
24444630.
316. Zhang L, Han DM. A brief introduction to non-allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010; 45:976–981. PMID:
21215044.
317. Wang H, Zhang J, You S, Ao Y, Bai Y, Shi H, et al. Diagnosis and clinical characteristics of patients with non-allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014; 49:501–505. PMID:
25241870.
318. Meng CD, Li L, Jiang XD, Dong Z, Zhu DD. Clinical characteristics in patients with non-allergic rhinitis and allergic rhinitis: preliminary analysis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010; 45:999–1002. PMID:
21215048.
319. Zhou XL, Long Y, Lin CZ, Jiang YS, Lu XD, Yang C, et al. Microbic distribution of acute rhinitis patient's nasal cavity and dependability research of respiratory infection. Chin J Prim Med Pharm. 2009; 16:437–438.
320. Zhang CD. Comparison of eosiniphis and total IgE in nasal secretion of allergic rhinitis and acute rhinitis patients. Med Lab Sci Clin. 2009; 20:63–64.
321. Wang S, Tang Q, Qian W, Fan Y. Meta-analysis of clinical trials on traditional Chinese herbal medicine for treatment of persistent allergic rhinitis. Allergy. 2012; 67:583–592. PMID:
22435619.

322. Aspirin ZY. Intolerance-rhinitis, sinusitis, nasal polyps and asthma. Clin Otorhinolaryngol (China). 2000; 14:381–383.
323. Lu M, Liu HB, Zhu WH, Chen BH, Liu Y, Zhao R, et al. Spontaneous cerebrospinal fluid rhinorrhea: case report. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2001; 36:69.
324. Huang DQ, Li WR, Ou XY. One case of posttraumatic cerebrospinal fluid rhinorrhea. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2006; 41:549. PMID:
17007387.
325. Ma F. 32 cases of nasal foreign body misdiagnosed as rhinitis. Clin Misdiagnosis Mistherapy. 2010; 23:94.
326. Jiang XD, Dong Z, Li GY, Gao G, Zhu DD. Endoscopic surgery for 89 cases of nasal inverted papilloma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010; 45:186–189. PMID:
20450694.
327. Dietz de Loos DA, Segboer CL, Gevorgyan A, Fokkens WJ. Disease-specific quality-of-life questionnaires in rhinitis and rhinosinusitis: review and evaluation. Curr Allergy Asthma Rep. 2013; 13:162–170. PMID:
23299562.

328. Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991; 21:77–83. PMID:
2021881.

329. Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of life in children with rhinoconjunctivitis. J Allergy Clin Immunol. 1998; 101:163–170. PMID:
9500748.

330. Juniper EF, Guyatt GH, Dolovich J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: development and testing of a questionnaire for clinical trials. J Allergy Clin Immunol. 1994; 93:413–423. PMID:
8120268.

331. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999; 104:364–369. PMID:
10452758.

332. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Development and validation of the mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin Exp Allergy. 2000; 30:132–140. PMID:
10606940.

333. Juniper EF, Rohrbaugh T, Meltzer EO. A questionnaire to measure quality of life in adults with nocturnal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2003; 111:484–490. PMID:
12642826.

334. Hellings PW, Fokkens WJ, Akdis C, Bachert C, Cingi C, Dietz de, et al. Uncontrolled allergic rhinitis and chronic rhinosinusitis: where do we stand today? Allergy. 2013; 68:1–7.

335. Nurmatov U, van Schayck CP, Hurwitz B, Sheikh A. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012; 67:158–165. PMID:
22103686.

336. Li Y, Cheng L, Chen X, Yang B, Wang D. Efficacy evaluation of a pollen blocker cream against dust-mite allergy: a multicenter, randomized, double-blind, placebo-controlled crossover trial. Am J Rhinol Allergy. 2015; 29:e129–e133. PMID:
26358336.

337. Jaakkola MS, Quansah R, Hugg TT, Heikkinen SA, Jaakkola JJ. Association of indoor dampness and molds with rhinitis risk: a systematic review and meta-analysis. J Allergy Clin Immunol. 2013; 132:1099–1110.e18. PMID:
24028857.
338. Kenney P, Hilberg O, Laursen AC, Peel RG, Sigsgaard T. Preventive effect of nasal filters on allergic rhinitis: a randomized, double-blind, placebo-controlled crossover park study. J Allergy Clin Immunol. 2015; 136:1566–1572.e5. PMID:
26141263.
339. Schwetz S, Olze H, Melchisedech S, Grigorov A, Latza R. Efficacy of pollen blocker cream in the treatment of allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2004; 130:979–984. PMID:
15313870.

340. Åberg N, Dahl Å, Benson M. A nasally applied cellulose powder in seasonal allergic rhinitis (SAR) in children and adolescents; reduction of symptoms and relation to pollen load. Pediatr Allergy Immunol. 2011; 22:594–599. PMID:
21645117.

341. Åberg N, Ospanova ST, Nikitin NP, Emberlin J, Dahl Å. A nasally applied cellulose powder in seasonal allergic rhinitis in adults with grass pollen allergy: a double-blind, randomized, placebo-controlled, parallel-group study. Int Arch Allergy Immunol. 2014; 163:313–318. PMID:
24852424.

342. Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011; 128:1139–1150.e4. PMID:
22035879.

343. Simons FE. Advances in H1-antihistamines. N Engl J Med. 2004; 351:2203–2217. PMID:
15548781.

344. Simons FE, Simons KJ. H1 antihistamines: current status and future directions. World Allergy Organ J. 2008; 1:145–155. PMID:
23282578.
345. Zhang L, Cheng L, Hong J. The clinical use of cetirizine in the treatment of allergic rhinitis. Pharmacology. 2013; 92:14–25. PMID:
23867423.

346. Simons FE. Early Prevention of Asthma in Atopic Children (EPAAC) Study Group. Safety of levocetirizine treatment in young atopic children: an 18-month study. Pediatr Allergy Immunol. 2007; 18:535–542. PMID:
17561929.

347. Nickels AS, Dimov V, Wolf R. Pharmacokinetic evaluation of olopatadine for the treatment of allergic rhinitis and conjunctivitis. Expert Opin Drug Metab Toxicol. 2011; 7:1593–1599. PMID:
22032416.

348. Horak F, Zieglmayer UP, Zieglmayer R, Kavina A, Marschall K, Munzel U, et al. Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. 2006; 22:151–157. PMID:
16393441.

349. Ratner PH, Findlay SR, Hampel F Jr, van Bavel J, Widlitz MD, Freitag JJ. A double-blind, controlled trial to assess the safety and efficacy of azelastine nasal spray in seasonal allergic rhinitis. J Allergy Clin Immunol. 1994; 94:818–825. PMID:
7963150.

350. Kaliner MA, Berger WE, Ratner PH, Siegel CJ. The efficacy of intranasal antihistamines in the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2011; 106(Suppl):S6–S11. PMID:
21277531.

351. Feng S, Deng C, Li L, Liao W, Fan Y, Xu G, et al. Efficacy of intranasal antihistamine in the treatment of allergic rhinitis: a meta-analysis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014; 49:832–838. PMID:
25567439.
352. Han D, Chen L, Cheng L, Liu S, Fu Z, Zhang W, et al. A multicenter randomized double-blind 2-week comparison study of azelastine nasal spray 0.1% versus levocabastine nasal spray 0.05% in patients with moderate-to-severe allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2011; 73:260–265. PMID:
21832863.

353. Cobanoğlu B, Toskala E, Ural A, Cingi C. Role of leukotriene antagonists and antihistamines in the treatment of allergic rhinitis. Curr Allergy Asthma Rep. 2013; 13:203–208. PMID:
23389557.

354. Kushnir NM. The role of decongestants, cromolyn, guafenesin, saline washes, capsaicin, leukotriene antagonists, and other treatments on rhinitis. Immunol Allergy Clin North Am. 2011; 31:601–617. PMID:
21737044.
355. Ouyang Y, Fan E, Li Y, Zhang L. Onset feature and efficacy of early interventional treatment of Artemisia pollinosis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014; 49:272–276. PMID:
24931013.
356. Hara H, Sugahara K, Hashimoto M, Mikuriya T, Tahara S, Yamashita H. Effectiveness of the leukotriene receptor antagonist pranlukast hydrate for the treatment of sleep disorder in patients with perennial allergic rhinitis. Acta Otolaryngol. 2014; 134:307–313. PMID:
24460152.

357. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014; 9:e112815. PMID:
25383622.

358. Liu X, Xing Z, Gao Z. The effect of antianaphylaxis drugs on specific IgE and eosinophil in serum of patients with allergic rhinitis. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005; 19:337–339. PMID:
16075981.
359. Pinar E, Eryigit O, Oncel S, Calli C, Yilmaz O, Yuksel H. Efficacy of nasal corticosteroids alone or combined with antihistamines or montelukast in treatment of allergic rhinitis. Auris Nasus Larynx. 2008; 35:61–66. PMID:
17826020.

360. Qu S, Li T, Chen Y, Lin Z, Ou Z. The role of leukotriene D4 antagonist in allergic rhinitis with steroid resistance. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005; 19:557–559. PMID:
16176012.
361. Liang M, Xu R, Xu G. Recent advances in allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015; 29:202–206. PMID:
26012287.
362. Panwankar R, Canonica GW, Holgate ST. WHO white book on allergy: update 2013. Milwaukee, WI: World Allergy Organization;2013.
363. Bascom R, Wachs M, Naclerio RM, Pipkorn U, Galli SJ, Lichtenstein LM. Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J Allergy Clin Immunol. 1988; 81:580–589. PMID:
2450113.

364. Pipkorn U, Proud D, Lichtenstein LM, Kagey-Sobotka A, Norman PS, Naclerio RM. Inhibition of mediator release in allergic rhinitis by pretreatment with topical glucocorticosteroids. N Engl J Med. 1987; 316:1506–1510. PMID:
2438553.

365. Erin EM, Leaker BR, Zacharasiewicz AS, Higgins LA, Williams TJ, Boyce MJ, et al. Single dose topical corticosteroid inhibits IL-5 and IL-13 in nasal lavage following grass pollen challenge. Allergy. 2005; 60:1524–1529. PMID:
16266385.

366. Shah SA, Berger RL, McDermott J, Gupta P, Monteith D, Connor A, et al. Regional deposition of mometasone furoate nasal spray suspension in humans. Allergy Asthma Proc. 2015; 36:48–57. PMID:
25562556.

367. Christodoulopoulos P, Cameron L, Durham S, Hamid Q. Molecular pathology of allergic disease. II: upper airway disease. J Allergy Clin Immunol. 2000; 105:211–223. PMID:
10669839.
368. Meltzer EO, Jalowayski AA, Orgel HA, Harris AG. Subjective and objective assessments in patients with seasonal allergic rhinitis: effects of therapy with mometasone furoate nasal spray. J Allergy Clin Immunol. 1998; 102:39–49. PMID:
9679846.

369. Igarashi T, Nakazato Y, Kunishige T, Fujita M, Yamada Y, Fujimoto C, et al. Mometasone furoate nasal spray relieves the ocular symptoms of seasonal allergic rhinoconjunctivitis. J Nippon Med Sch. 2012; 79:182–189. PMID:
22791118.

370. Bernstein DI, Levy AL, Hampel FC, Baidoo CA, Cook CK, Philpot EE, et al. Treatment with intranasal fluticasone propionate significantly improves ocular symptoms in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2004; 34:952–957. PMID:
15196285.

371. Bhatia S, Baroody FM, deTineo M, Naclerio RM. Increased nasal airflow with budesonide compared with desloratadine during the allergy season. Arch Otolaryngol Head Neck Surg. 2005; 131:223–228. PMID:
15781762.

372. Bielory L, Chun Y, Bielory BP, Canonica GW. Impact of mometasone furoate nasal spray on individual ocular symptoms of allergic rhinitis: a meta-analysis. Allergy. 2011; 66:686–693. PMID:
21261661.

373. Zhang L, Xu G, Wang X, Liu S, Li Y, Wang S, et al. Mometasone furoate nasal spray reduces symptoms and improves quality of life in Chinese patients with moderate to severe allergic rhinitis: a multicenter open-label study. Acta Otolaryngol. 2009; 129:1463–1468. PMID:
19922098.

374. Han D, Liu S, Zhang Y, Wang J, Wang D, Kong W, et al. Efficacy and safety of fluticasone furoate nasal spray in Chinese adult and adolescent subjects with intermittent or persistent allergic rhinitis. Allergy Asthma Proc. 2011; 32:472–481. PMID:
21888757.

375. Cheng L. Prophylactic treatment for seasonal allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013; 48:532–534. PMID:
24313198.
376. Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016; 51:6–24. PMID:
26791765.
377. Song XH, Zhang L, Han DM, Wang KJ, Wang H, Zhang W. Effects of oxymetazoline hydrochloride on ex vivo human nasal cilia movement measured with high-speed digital microscopy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008; 43:268–271. PMID:
18666692.
378. Meltzer EO, Bernstein DI, Prenner BM, Berger WE, Shekar T, Teper AA. Mometasone furoate nasal spray plus oxymetazoline nasal spray: short-term efficacy and safety in seasonal allergic rhinitis. Am J Rhinol Allergy. 2013; 27:102–108. PMID:
23562197.

379. Slavin RG. Special considerations in treatment of allergic rhinitis in the elderly: role of intranasal corticosteroids. Allergy Asthma Proc. 2010; 31:179–184. PMID:
20615319.

380. Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Subspecialty Group of Rhinology and Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Editorial Board of Chinese Journal of Pediatrics. Guidelines for diagnosis and treatment of pediatric allergic rhinitis (2010, Chongqing). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 46:7–8. PMID:
21429322.
381. Garavello W, Somigliana E, Acaia B, Gaini L, Pignataro L, Gaini RM. Nasal lavage in pregnant women with seasonal allergic rhinitis: a randomized study. Int Arch Allergy Immunol. 2010; 151:137–141. PMID:
19752567.

382. Georgitis JW. Nasal hyperthermia and simple irrigation for perennial rhinitis. Changes in inflammatory mediators. Chest. 1994; 106:1487–1492. PMID:
7956408.
383. Ponikau JU, Sherris DA, Kephart GM, Kern EB, Congdon DJ, Adolphson CR, et al. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005; 116:362–369. PMID:
16083791.

384. Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope. 1997; 107:500–503. PMID:
9111380.

385. Boek WM, Graamans K, Natzijl H, van Rijk PP, Huizing EH. Nasal mucociliary transport: new evidence for a key role of ciliary beat frequency. Laryngoscope. 2002; 112:570–573. PMID:
12148873.

386. Li H, Sha Q, Zuo K, Jiang H, Cheng L, Shi J, et al. Nasal saline irrigation facilitates control of allergic rhinitis by topical steroid in children. ORL J Otorhinolaryngol Relat Spec. 2009; 71:50–55. PMID:
19047814.

387. Sun YM. 47 cases of clinical observation on kidney yang-deficiency type of allergic rhinitis with Yougui soup treatment. Tradit Chin Med Rev. 2004; 10:40–41.
388. Lin HW, Shi ZX, Ma SM. Clinical study on the treatment of allergic rhinitis by replenishing qi and consolidating the exterior. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1989; 9:2631.
389. Qiu Baoshan LP, Ma L. Experimental study of the correlation between allergic rhinitis and spleen deficiency. J Tradit Chin Med. 2003; 21:10402–10411.
390. Qiu Baoshan LP, Huang K. The effect of benefiting qi decoction on spleen deficiency type of allergic rhinitis. Tradit Chin Med Clin Pharmacol. 2003; 14:1472–1491.
391. Yuehong Liao AO, Xiang J. Clinical observation on the therapeutic effect of Metal-clearing methodon allergic rhinitis and a study on the semeiological basis for this kind of therapy. Chin Otorhinolaryngol J Integr Med. 2007; 15:427–429.
392. Chan RY, Chien WT. The effects of two Chinese herbal medicinal formulae vs. placebo controls for treatment of allergic rhinitis: a randomised controlled trial. Trials. 2014; 15:261. PMID:
24986270.

393. Min C, Peng C, Wei G, Huang X, Fu T, Du Y, et al. Moxibustion with Chinese herbal has good effect on allergic rhinitis. Int J Clin Exp Med. 2015; 8:16480–16487. PMID:
26629174.
394. Zhao Y, Woo KS, Ma KH, van Hansselt CA, Wong KC, Cheng KF, et al. Treatment of perennial allergic rhinitis using Shi-Bi-Lin, a Chinese herbal formula. J Ethnopharmacol. 2009; 122:100–105. PMID:
19118617.

395. Chui SH, Shek SL, Fong MY, Szeto YT, Chan K. A panel study to evaluate quality of life assessments in patients suffering from allergic rhinitis after treatment with a Chinese herbal nasal drop. Phytother Res. 2010; 24:609–613. PMID:
20014162.

396. Hsu WH, Ho TJ, Huang CY, Ho HC, Liu YL, Liu HJ, et al. Chinese medicine acupoint herbal patching for allergic rhinitis: a randomized controlled clinical trial. Am J Chin Med. 2010; 38:661–673. PMID:
20626052.

397. Jung JW, Kang HR, Ji GE, Park MS, Song WJ, Kim MH, et al. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res. 2011; 3:103–110. PMID:
21461249.

398. Hu G, Walls RS, Bass D, Ramon B, Grayson D, Jones M, et al. The Chinese herbal formulation biminne in management of perennial allergic rhinitis: a randomized, double-blind, placebo-controlled, 12-week clinical trial. Ann Allergy Asthma Immunol. 2002; 88:478–487. PMID:
12027069.

399. Lenon GB, Li CG, Da Costa C, Thien FC, Shen Y, Xue CC. Lack of efficacy of a herbal preparation (RCM-102) for seasonal allergic rhinitis: a double blind, randomised, placebo-controlled trial. Asia Pac Allergy. 2012; 2:187–194. PMID:
22872821.

400. Brinkhaus B, Hummelsberger J, Kohnen R, Seufert J, Hempen CH, Leonhardy H, et al. Acupuncture and Chinese herbal medicine in the treatment of patients with seasonal allergic rhinitis: a randomized-controlled clinical trial. Allergy. 2004; 59:953–960. PMID:
15291903.

401. Zhang X, Lan F, Zhang Y, Zhang L. Chinese herbal medicine to treat allergic rhinitis: evidence from a meta-analysis. Allergy Asthma Immunol Res. 2018; 10:34–42. PMID:
29178676.

402. Xue CC, Thien FC, Zhang JJ, Yang W, Da Costa C, Li CG. Effect of adding a Chinese herbal preparation to acupuncture for seasonal allergic rhinitis: randomised double-blind controlled trial. Hong Kong Med J. 2003; 9:427–434. PMID:
14660810.
403. Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011; 127:18–27. PMID:
21211639.

404. Tarzi M, Klunker S, Texier C, Verhoef A, Stapel SO, Akdis CA, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006; 36:465–474. PMID:
16630151.

405. Alexander C, Ying S, B Kay A, Larché M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005; 35:52–58. PMID:
15649266.
406. Woo HY, Kim YS, Kang NI, Chung WC, Song CH, Choi IW, et al. Mechanism for acute oral desensitization to antibiotics. Allergy. 2006; 61:954–958. PMID:
16867049.

407. Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010; 65:1558–1565. PMID:
20584008.

408. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006; 6:761–771. PMID:
16998509.

409. Lambrecht BN, Pauwels RA, Fazekas De St Groth B. Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J Immunol. 2000; 164:2937–2946. PMID:
10706680.

410. Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000; 192:1213–1222. PMID:
11067871.

411. de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004; 200:89–98. PMID:
15238608.

412. Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998; 102:98–106. PMID:
9649562.

413. Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004; 172:3252–3259. PMID:
14978133.

414. Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004; 199:1567–1575. PMID:
15173208.

415. Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004; 363:608–615. PMID:
14987885.

416. Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003; 33:1205–1214. PMID:
12731045.
417. Verhoef A, Alexander C, Kay AB, Larché M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med. 2005; 2:e78. PMID:
15783262.

418. Varney VA, Hamid QA, Gaga M, Ying S, Jacobson M, Frew AJ, et al. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest. 1993; 92:644–651. PMID:
8349803.

419. Ebner C, Siemann U, Bohle B, Willheim M, Wiedermann U, Schenk S, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997; 27:1007–1015. PMID:
9678832.
420. Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Müller U, et al. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15
in vitro. J Clin Invest. 1996; 98:1676–1683. PMID:
8833918.
421. Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003; 111:1255–1261. PMID:
12789226.

422. Akdis CA, Blaser K. IL-10-induced anergy in peripheral T cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. FASEB J. 1999; 13:603–609. PMID:
10094921.

423. Bellinghausen I, Metz G, Enk AH, Christmann S, Knop J, Saloga J. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. Eur J Immunol. 1997; 27:1131–1139. PMID:
9174602.

424. Pereira EA, Silva DA, Cunha-Júnior JP, Almeida KC, Alves R, Sung SJ, et al. IgE, IgG1, and IgG4 antibody responses to Blomia tropicalis in atopic patients. Allergy. 2005; 60:401–406. PMID:
15679730.
425. Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy (hay fever). J Exp Med. 1935; 62:733–750. PMID:
19870445.

426. Lichtenstein LM, Holtzman NA, Burnett LS. A quantitative
in vitro study of the chromatographic distribution and immunoglobulin characteristics of human blocking antibody. J Immunol. 1968; 101:317–324. PMID:
4174497.
427. Golden DB, Meyers DA, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Clinical relevance of the venom-specific immunoglobulin G antibody level during immunotherapy. J Allergy Clin Immunol. 1982; 69:489–493. PMID:
7076990.

428. Müller U, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy. 1989; 44:412–418. PMID:
2802114.

429. Djurup R, Malling HJ. High IgG4 antibody level is associated with failure of immunotherapy with inhalant allergens. Clin Allergy. 1987; 17:459–468. PMID:
3677372.

430. King TP, Jim SY, Monsalve RI, Kagey-Sobotka A, Lichtenstein LM, Spangfort MD. Recombinant allergens with reduced allergenicity but retaining immunogenicity of the natural allergens: hybrids of yellow jacket and paper wasp venom allergen antigen 5s. J Immunol. 2001; 166:6057–6065. PMID:
11342623.

431. Linhart B, Hartl A, Jahn-Schmid B, Verdino P, Keller W, Krauth MT, et al. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005; 115:1010–1016. PMID:
15867859.

432. Hirahara K, Tatsuta T, Takatori T, Ohtsuki M, Kirinaka H, Kawaguchi J, et al. Preclinical evaluation of an immunotherapeutic peptide comprising 7 T-cell determinants of Cry j 1 and Cry j 2, the major Japanese cedar pollen allergens. J Allergy Clin Immunol. 2001; 108:94–100. PMID:
11447388.

433. Soyer OU, Akdis M, Akdis CA. Mechanisms of subcutaneous allergen immunotherapy. Immunol Allergy Clin North Am. 2011; 31:175–190. PMID:
21530813.

434. Moote W, Kim H. Allergen-specific immunotherapy. Allergy Asthma Clin Immunol. 2011; 7(Suppl 1):S5. PMID:
22166078.

435. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127(Suppl):S1–S55. PMID:
21122901.
436. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015; 136:556–568. PMID:
26162571.

437. Zhang L, Wang C, Han D, Wang X, Zhao Y, Liu J. Comparative study of cluster and conventional immunotherapy schedules with dermatophagoides pteronyssinus in the treatment of persistent allergic rhinitis. Int Arch Allergy Immunol. 2009; 148:161–169. PMID:
18802361.
438. Tabar AI, Echechipía S, García BE, Olaguibel JM, Lizaso MT, Gómez B, et al. Double-blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 2005; 116:109–118. PMID:
15990782.

439. Lou W, Wang C, Wang Y, Han D, Zhang L. Enhancement of the frequency and function of IL-10-secreting type I T regulatory cells after 1 year of cluster allergen-specific immunotherapy. Int Arch Allergy Immunol. 2012; 159:391–398. PMID:
22846686.

440. Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014; 7:6. PMID:
24679069.

441. Sikora JM, Tankersley MS. ACAAI Immunotherapy and Diagnostics Committee. Perception and practice of sublingual immunotherapy among practicing allergists in the United States: a follow-up survey. Ann Allergy Asthma Immunol. 2013; 110:194–197.e4. PMID:
23548531.

442. Lin SY, Erekosima N, Kim JM, Ramanathan M, Suarez-Cuervo C, Chelladurai Y, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013; 309:1278–1288. PMID:
23532243.
443. Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011; 66:740–752. PMID:
21443635.

444. Meng Q, Liu X, Li P, He L, Xie J, Gao X, et al. The influence of house dust mite sublingual immunotherapy on the TSLP-OX40L signaling pathway in patients with allergic rhinitis. Int Forum Allergy Rhinol. 2016; 6:862–870. PMID:
27012942.

445. Luo X, Hong H, Tang J, Wu X, Lin Z, Ma R, et al. Increased expression of miR-146a in children with allergic rhinitis after allergen-specific immunotherapy. Allergy Asthma Immunol Res. 2016; 8:132–140. PMID:
26739406.

446. Lin Z, Liu Q, Li T, Chen D, Chen D, Xu R. The effects of house dust mite sublingual immunotherapy in patients with allergic rhinitis according to duration. Int Forum Allergy Rhinol. 2016; 6:82–87. PMID:
26575696.

447. Xu CX, Zhang ML, Li BZ, He Y, Zou ZH, Wu QR, et al. Efficacy of sublingual immunotherapy with dermatophagoides farinae extract in monosensitized and polysensitized patients with allergic rhinitis: clinical observation and analysis. Biomed Res Int. 2015; 2015:187620. PMID:
26000283.
448. Aboshady OA, Elghanam KM. Sublingual immunotherapy in allergic rhinitis: efficacy, safety, adherence and guidelines. Clin Exp Otorhinolaryngol. 2014; 7:241–249. PMID:
25436040.

449. Wang C, Zhang L. Specific immunotherapy for allergic rhinitis in children. Curr Opin Otolaryngol Head Neck Surg. 2014; 22:487–494. PMID:
25207858.

450. Prigal SJ. A ten-year study of repository injections of allergens: local reactions and their management. Ann Allergy. 1972; 30:529–535. PMID:
5056915.
451. Nelson BL, Dupont LA, Reid MJ. Prospective survey of local and systemic reactions to immunotherapy with pollen extracts. Ann Allergy. 1986; 56:331–334. PMID:
3963525.
452. Tankersley MS, Butler KK, Butler WK, Goetz DW. Local reactions during allergen immunotherapy do not require dose adjustment. J Allergy Clin Immunol. 2000; 106:840–843. PMID:
11080704.

453. Kelso JM. The rate of systemic reactions to immunotherapy injections is the same whether or not the dose is reduced after a local reaction. Ann Allergy Asthma Immunol. 2004; 92:225–227. PMID:
14989390.

454. Zhu L, Lu JH, Xie Q, Wu YL, Zhu LP, Cheng L. Compliance and safety evaluation of subcutaneous versus sublingual immunotherapy in mite-sensitized patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010; 45:444–449. PMID:
21055319.
455. Calabria CW, Stolfi A, Tankersley MS. The REPEAT study: recognizing and evaluating periodic local reactions in allergen immunotherapy and associated systemic reactions. Ann Allergy Asthma Immunol. 2011; 106:49–53. PMID:
21195945.

456. Coop CA, Tankersley MS. Patient perceptions regarding local reactions from allergen immunotherapy injections. Ann Allergy Asthma Immunol. 2008; 101:96–100. PMID:
18681091.

457. Passalacqua G, Baena-Cagnani CE, Bousquet J, Canonica GW, Casale TB, Cox L, et al. Grading local side effects of sublingual immunotherapy for respiratory allergy: speaking the same language. J Allergy Clin Immunol. 2013; 132:93–98. PMID:
23683513.
458. Wen CJ, Zhu MF, Ren WM, Liu XY, Qian H. Clinical efficacy and safety of sublingual immunotherapy using standardized Dermatophagoides farinae extract for children with combined allergic rhinitis and asthma syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 46:393–396. PMID:
21781561.
459. Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the world allergy organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010; 125:569–574. 574.e1–574.e7. PMID:
20144472.

460. Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008–2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract. 2014; 2:161–167. PMID:
24607043.

461. Bernstein DI, Wanner M, Borish L, Liss GM. Immunotherapy Committee, American Academy of Allergy, Asthma and Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol. 2004; 113:1129–1136. PMID:
15208595.

462. Chen J, Li B, Zhao Y, Zhang Q, Wan L, Liu J, et al. A prospective multicenter study of systemic reactions in standardized specific immunotherapy for allergic rhinitis in China. Am J Rhinol Allergy. 2014; 28:e40–e44. PMID:
24717880.

463. Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006; 117:169–175. PMID:
16387602.

464. Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006; 117:1021–1035. PMID:
16675328.

465. Berchtold E, Maibach R, Müller U. Reduction of side effects from rush-immunotherapy with honey bee venom by pretreatment with terfenadine. Clin Exp Allergy. 1992; 22:59–65. PMID:
1551035.

466. Nielsen L, Johnsen CR, Mosbech H, Poulsen LK, Malling HJ. Antihistamine premedication in specific cluster immunotherapy: a double-blind, placebo-controlled study. J Allergy Clin Immunol. 1996; 97:1207–1213. PMID:
8648014.

467. Wöhrl S, Gamper S, Hemmer W, Heinze G, Stingl G, Kinaciyan T. Premedication with montelukast reduces local reactions of allergen immunotherapy. Int Arch Allergy Immunol. 2007; 144:137–142. PMID:
17536222.

468. Scranton SE, Gonzalez EG, Waibel KH. Incidence and characteristics of biphasic reactions after allergen immunotherapy. J Allergy Clin Immunol. 2009; 123:493–498. PMID:
19064282.

469. Kemp SF, Lockey RF, Simons FE. World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy. 2008; 63:1061–1070. PMID:
18691308.

470. Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy. 2006; 61(Suppl 82):1–20.

471. Tan G, Ma Y, Li H, Li W, Wang J. Long-term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2012; 138:492–497. PMID:
22652948.

472. Jang TY, Kim YH, Shin SH. Long-term effectiveness and safety of endoscopic vidian neurectomy for the treatment of intractable rhinitis. Clin Exp Otorhinolaryngol. 2010; 3:212–216. PMID:
21217963.

473. Kobayashi T, Hyodo M, Nakamura K, Komobuchi H, Honda N. Resection of peripheral branches of the posterior nasal nerve compared to conventional posterior neurectomy in severe allergic rhinitis. Auris Nasus Larynx. 2012; 39:593–596. PMID:
22341334.

474. Ogawa T, Takeno S, Ishino T, Hirakawa K. Submucous turbinectomy combined with posterior nasal neurectomy in the management of severe allergic rhinitis: clinical outcomes and local cytokine changes. Auris Nasus Larynx. 2007; 34:319–326. PMID:
17433591.

475. Wu AW, Ting JY. Indications for surgery in refractory rhinitis. Curr Allergy Asthma Rep. 2014; 14:414. PMID:
24408537.

476. Li PZ, Gu DS, Lu MP, Li YJ, Shen Y, Cheng L. Nasal coblation plasma surgery for the treatment of persistent allergic rhinitis: an evaluation of short-term outcomes. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013; 48:891–894. PMID:
24444632.
477. Cassano M, Granieri C, Del Giudice AM, Mora F, Fiocca-Matthews E, Cassano P. Restoration of nasal cytology after endoscopic turbinoplasty versus laser-assisted turbinoplasty. Am J Rhinol Allergy. 2010; 24:310–314. PMID:
20819472.

478. Caffier PP, Scherer H, Neumann K, Lück S, Enzmann H, Haisch A. Diode laser treatment in therapy-resistant allergic rhinitis: impact on nasal obstruction and associated symptoms. Lasers Med Sci. 2011; 26:57–67. PMID:
20623243.

479. Chen YL, Tan CT, Huang HM. Long-term efficacy of microdebrider-assisted inferior turbinoplasty with lateralization for hypertrophic inferior turbinates in patients with perennial allergic rhinitis. Laryngoscope. 2008; 118:1270–1274. PMID:
18401269.

480. Lin HC, Lin PW, Friedman M, Chang HW, Su YY, Chen YJ, et al. Long-term results of radiofrequency turbinoplasty for allergic rhinitis refractory to medical therapy. Arch Otolaryngol Head Neck Surg. 2010; 136:892–895. PMID:
20644029.

481. Lee JY, Lee JD. Comparative study on the long-term effectiveness between coblation- and microdebrider-assisted partial turbinoplasty. Laryngoscope. 2006; 116:729–734. PMID:
16652079.

482. Tan GL, Ma YH, Liu GS, Wang JJ, Li W. Therapeutic effect of endoscopic vidian neurectomy on moderate-severe persistent allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 46:449–454. PMID:
21924093.
483. Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014; 133:1521–1534. PMID:
24433703.

484. Daoud A, Xie Z, Ma Y, Wang T, Tan G. Changes of T-helper type 1/2 cell balance by anticholinergic treatment in allergic mice. Ann Allergy Asthma Immunol. 2014; 112:249–255. PMID:
24428969.
485. Golding-Wood PH. Observations on petrosal and vidian neurectomy in chronic vasomotor rhinitis. J Laryngol Otol. 1961; 75:232–247. PMID:
13706533.

486. Lee JC, Kao CH, Hsu CH, Lin YS. Endoscopic transsphenoidal vidian neurectomy. Eur Arch Otorhinolaryngol. 2011; 268:851–856. PMID:
21221616.

487. Su WF, Liu SC, Chiu FS, Lee CH. Antegrade transsphenoidal vidian neurectomy: short-term surgical outcome analysis. Am J Rhinol Allergy. 2011; 25:e217–e220. PMID:
22185728.

488. Ikeda K, Yokoi H, Saito T, Kawano K, Yao T, Furukawa M. Effect of resection of the posterior nasal nerve on functional and morphological changes in the inferior turbinate mucosa. Acta Otolaryngol. 2008; 128:1337–1341. PMID:
18607960.

489. Liu HT, Ma RX, Yan XH, Feng NY, Zhou Y, Shen Y, et al. Clinical study on resection of the posterior nasal nerve for hyperreactive rhinopathy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013; 48:1032–1034. PMID:
24507005.
490. Shakeel M, Trinidade A, Ah-See KW. Complementary and alternative medicine use by otolaryngology patients: a paradigm for practitioners in all surgical specialties. Eur Arch Otorhinolaryngol. 2010; 267:961–971. PMID:
19771443.

491. Shakeel M, Trinidade A, Jehan S, Ah-See KW. The use of complementary and alternative medicine by patients attending a general otolaryngology clinic: can we afford to ignore it? Am J Otolaryngol. 2010; 31:252–260. PMID:
20015757.

492. Choi SM, Park JE, Li SS, Jung H, Zi M, Kim TH, et al. A multicenter, randomized, controlled trial testing the effects of acupuncture on allergic rhinitis. Allergy. 2013; 68:365–374. PMID:
23253122.

493. Brinkhaus B, Witt CM, Jena S, Liecker B, Wegscheider K, Willich SN. Acupuncture in patients with allergic rhinitis: a pragmatic randomized trial. Ann Allergy Asthma Immunol. 2008; 101:535–543. PMID:
19055209.

494. Chen Y, Jin X, Yu M, Qiu H, Fang Y, Zhang S, et al. Efficacy of acupuncture on moderate and severe allergic rhinitis. Zhongguo Zhen Jiu. 2015; 35:339–343. PMID:
26054141.
495. Chen S, Wang J, Bai P, Zhao Q, Tan C, Wang B, et al. Moderate and severe persistent allergic rhinitis treated with acupuncture: a randomized controlled trial. Zhongguo Zhen Jiu. 2015; 35:1209–1213. PMID:
26964157.
496. He TY, Li HQ, Zhao YD, Gao HY. Treatment of 60 cases of allergic rhinitis mainly with point-through-point method. Zhongguo Zhen Jiu. 2006; 26:110–112. PMID:
16541860.
497. Zhang YQ. Clinical experience in acupuncture treatment of allergic rhinitis. J Tradit Chin Med. 2009; 29:186–189. PMID:
19894382.

498. Chen ZX. Clinical observation on acupuncture for treatment of allergic rhinitis. Zhongguo Zhenjiu. 2007; 27:578–580.
499. Petti FB, Liguori A, Ippoliti F. Study on cytokines IL-2, IL-6, IL-10 in patients of chronic allergic rhinitis treated with acupuncture. J Tradit Chin Med. 2002; 22:104–111. PMID:
12125480.
500. Liu TS, Qiu R, Lai XS. Efficacy on perennial allergic rhinitis treated with acupuncture at three nasal poinits and the acupoints selected by syndrome differentiation. Zhongguo Zhen Jiu. 2014; 34:1083–1086. PMID:
25675568.
501. Han D, Liu C, Qie L, Wang F, Wang Z. Acupoint selection and medication rules analysis for allergic rhinitis treated with acupoint application-based on data mining technology. Zhongguo Zhen Jiu. 2015; 35:1177–1180. PMID:
26939343.
502. Xie Y, Wan W, Zhao Y, Ye Z, Chen H, Hong X, et al. Impacts on the life quality of the patients with allergic rhinitis treated with warming acupuncture in winter and summer. Zhongguo Zhen Jiu. 2015; 35:1215–1220. PMID:
26964159.
503. Ou WX, Luo QY, Lin QM, Lin XH, Cao YM, Ma XW, et al. Efficacy observation on Jin's three-needle therapy for allergic rhinitis of lung qi deficiency and cold syndrome. Zhongguo Zhen Jiu. 2014; 34:445–448. PMID:
25022113.
504. Wang H, Li W, Ju XF, Yu XG. Effect of penetrating needling at head acupoints on perennial allergic rhinitis. Zhongguo Zhen Jiu. 2013; 33:789–792. PMID:
24298766.
505. Rao YQ, Han NY. Therapeutic effect of acupuncture on allergic rhinitis and its effects on immunologic function. Zhongguo Zhen Jiu. 2006; 26:557–560. PMID:
16941973.
506. Wang K, Chen L, Wang Y, Wang C, Zhang L. Sphenopalatine ganglion acupuncture improves nasal ventilation and modulates autonomic nervous activity in healthy volunteers: a randomized controlled study. Sci Rep. 2016; 6:29947. PMID:
27425415.

507. Zhang L, Yang W, Wang KJ. Acupuncture at ganglion pterygoplatinum for 71 cases of chronic simple rhinitis. Zhongguo Zhen Jiu. 2013; 33:495–496. PMID:
23967633.
508. Xinwu L. The mechanism analysis of treating nasal disease by sphenopalatine ganglion (acupoint “ZhiBi 3”) stimulation with acupuncture needle and an introduction to the relevant needling method. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 21:193–196.
509. Zhang H, Zhao M, Fu L. Short effect and adverse reaction of dog days plaster for allergic rhinitis. Zhongguo Zhen Jiu. 2016; 36:33–36. PMID:
26946731.
510. Chen J, Deng GZ, Chen F, Zhang SJ, Guo YF, Chen JQ, et al. Clinical comparative study on the influence of acupoint sticking therapy in dog days and in non-dog days to the quality of life of allergic rhinitis patients. Zhongguo Zhen Jiu. 2012; 32:31–34. PMID:
22295819.
511. Fang MS, Dou YC, Yao SM. Clinical research of medicinal vesiculation for perennial allergic rhinitis. Zhongguo Zhen Jiu. 2014; 34:857–860. PMID:
25509731.
512. Tang ZM, Chen JX, Tan JS. Therapy of cantharides extract for perennial allergic rhinitis and its effect on total IgE in serum. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995; 15:334–336. PMID:
7549381.
513. Cao W, Qiao P, Pang W, Liu M, Li A. Triple-strong stimulation therapy at Dazhui (GV 14) in prevention and treatment of children allergic rhinitis: a randomized controlled trial. Zhongguo Zhen Jiu. 2015; 35:38–42. PMID:
25906565.
514. Ke ZH, Long SH. Medicinal vesiculation combined with quick cupping at Shenque (CV 8) for allergic rhinitis with syndrome of yang deficiency: a randomized controlled trial. Zhongguo Zhen Jiu. 2014; 34:853–856. PMID:
25509730.
515. Chen C, Li YC, Qiu BS, Huang XP, Zhuang LX. Observation of long-term efficacy and life quality in allergic rhinitis treated with acupoint catgut embedding therapy combined with acupuncture-moxibustion therapy. Zhongguo Zhen Jiu. 2014; 34:439–443. PMID:
25022111.
516. Li XR, Zhang QX, Liu M, Chen Q, Liu Y, Zhang FB, et al. Catgut implantation at acupoints for allergic rhinitis: a systematic review. Chin J Integr Med. 2014; 20:235–240. PMID:
24615216.

517. Liang C, Jiang T. Acupoint autohemotherapy for allergic rhinitis and its effect on serum IL-12 and IFN-gamma. Zhongguo Zhen Jiu. 2012; 32:1077–1080. PMID:
23301470.
518. Li F, Liu KJ. Point-injection combined with TDP radiation for treatment of 30 cases of allergic rhinitis. Zhongguo Zhen Jiu. 2006; 26:25–26. PMID:
16491753.
519. Zhu LP, Zhang QZ, Shimada T, Enomoto T, Cheng L. Anti-allergic effects of the probiotic preparations of enterococcus on experimental allergic rhinitis in mice. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013; 48:555–562. PMID:
24313204.
520. Fan J, Hou Y, Zhou S, Cai X. Effect of Bifidobacterium on the immunity in BALB/c mice. Wei Sheng Wu Xue Bao. 2015; 55:484–491. PMID:
26211323.
521. Li SR, Wang HH, Wu ZY, Liu RH, Tong MH, Wang CH, et al. Efficacies of lactulose plus live combined Bacillus subtilis and Enterococcus faecium capsules in the treatment of functional constipation: a multicenter, randomized, double blind, controlled trial. Zhonghua Yi Xue Za Zhi. 2012; 92:2955–2960. PMID:
23328283.
522. Investigating Group for Prevention of AAD in Children with Pneumonia by Clostridium Butyricum and Bifidobacterium. Multicenter, randomized, controlled clinical trial on preventing antibiotic-associated diarrhea in children with pneumonia using the live Clostridium butyricum and Bifidobacterium combined Powder. Zhonghua Er Ke Za Zhi. 2012; 50:732–736. PMID:
23302558.
523. Soh SE, Aw M, Gerez I, Chong YS, Rauff M, Ng YP, et al. Probiotic supplementation in the first 6 months of life in at risk Asian infants--effects on eczema and atopic sensitization at the age of 1 year. Clin Exp Allergy. 2009; 39:571–578. PMID:
19134020.
524. West CE. Probiotics for allergy prevention. Benef Microbes. 2016; 7:171–179. PMID:
26689229.

525. Rautava S, Kalliomäki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002; 109:119–121. PMID:
11799376.

526. Chu Z, Zhang X, Meng B. Value of patient education in the treatment of allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015; 29:396–399. PMID:
26103654.
527. Scadding GK. Optimal management of allergic rhinitis. Arch Dis Child. 2015; 100:576–582. PMID:
25838332.

528. Wang K, Wang C, Xi L, Zhang Y, Ouyang Y, Lou H, et al. A randomized controlled trial to assess adherence to allergic rhinitis treatment following a daily short message service (SMS) via the mobile phone. Int Arch Allergy Immunol. 2014; 163:51–58. PMID:
24248037.

529. Li C, Xu P, Xu H, Zhu H. Evaluation on the immunotherapy efficacies of synthetic peptide vaccines in asthmatic mice with group I and II allergens from Dermatophagoides pteronyssinus. Int J Clin Exp Med. 2015; 8:20402–20412. PMID:
26884956.
530. Ou J, Shi W, Xu Y, Tao Z. Intranasal immunization with DNA vaccine coexpressing Der p 1 and ubiquitin in an allergic rhinitis mouse model. Ann Allergy Asthma Immunol. 2014; 113:658–665.e1. PMID:
25240330.

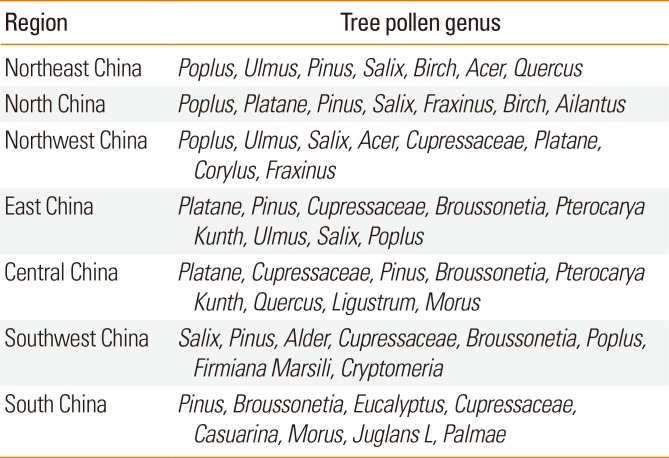
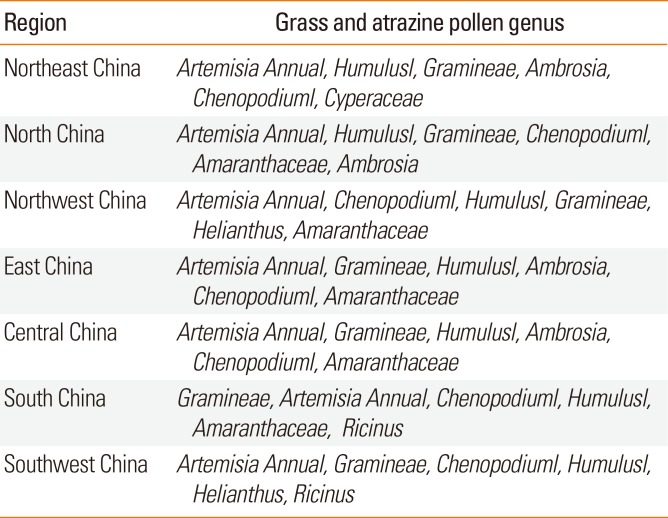
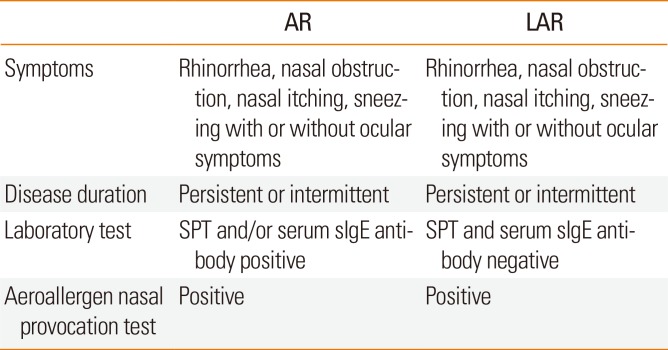
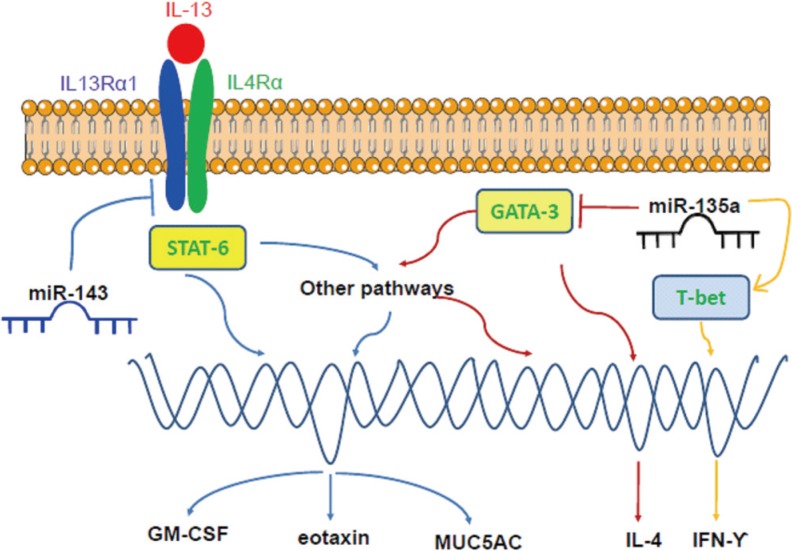
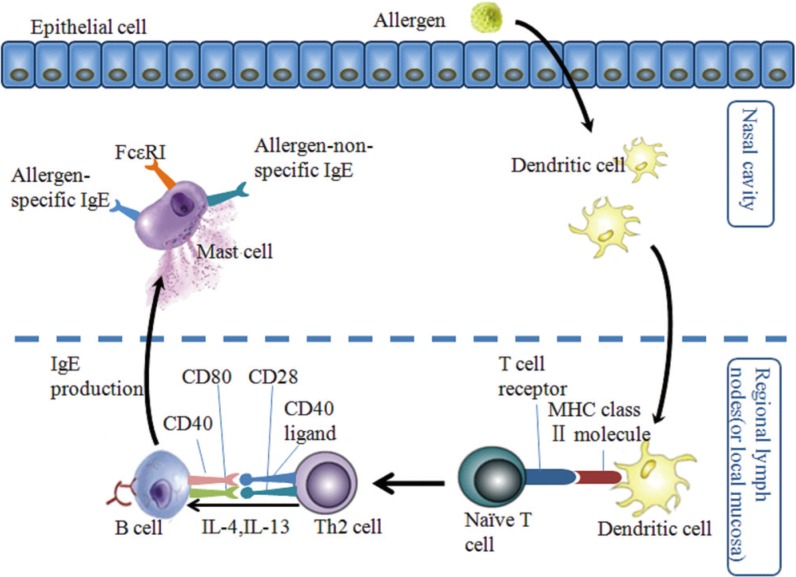
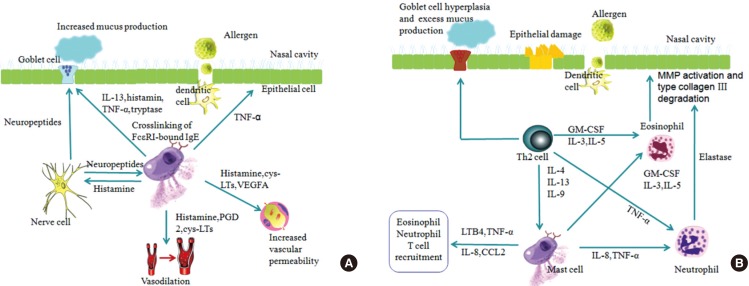
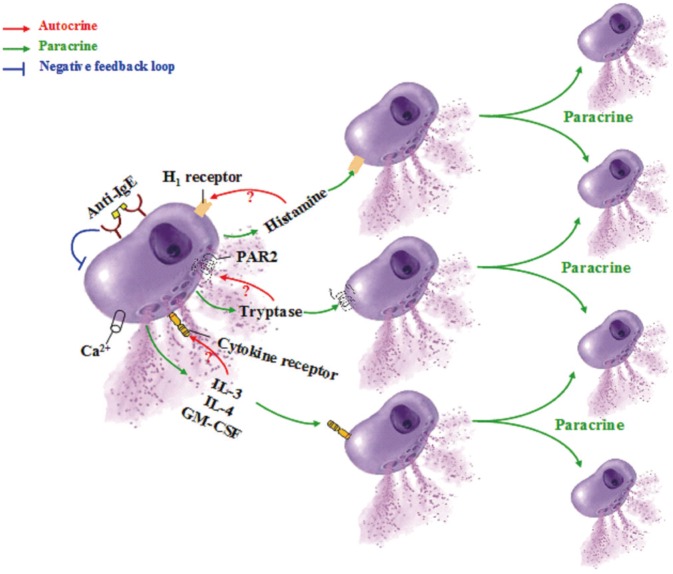
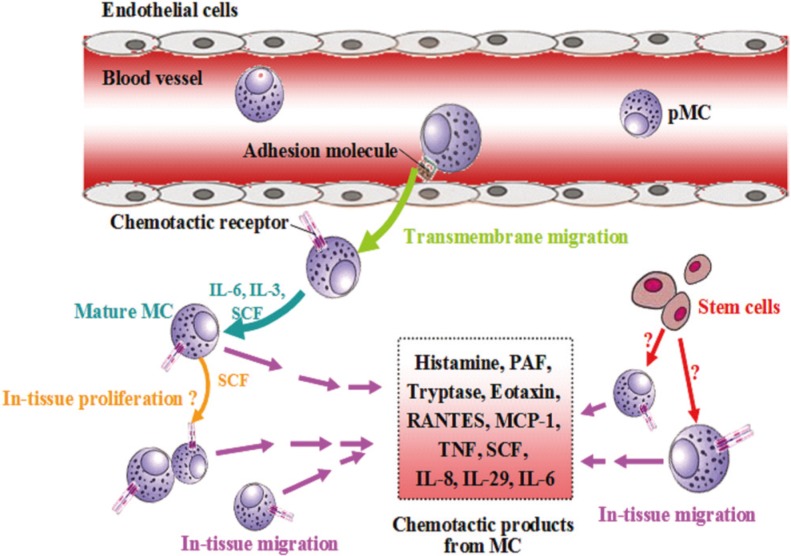
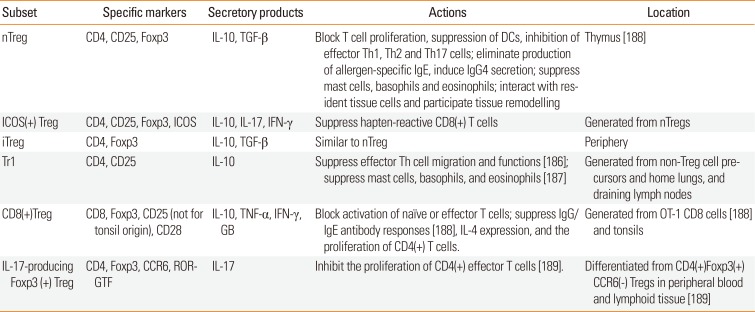
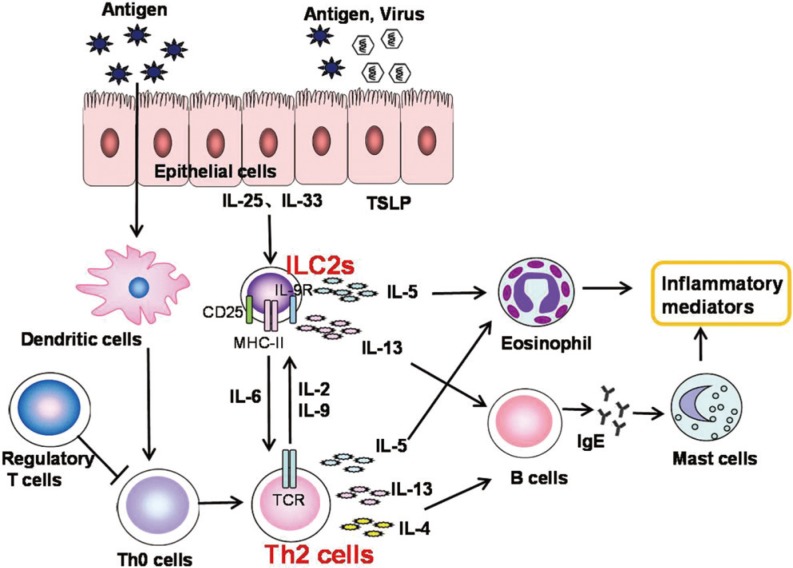
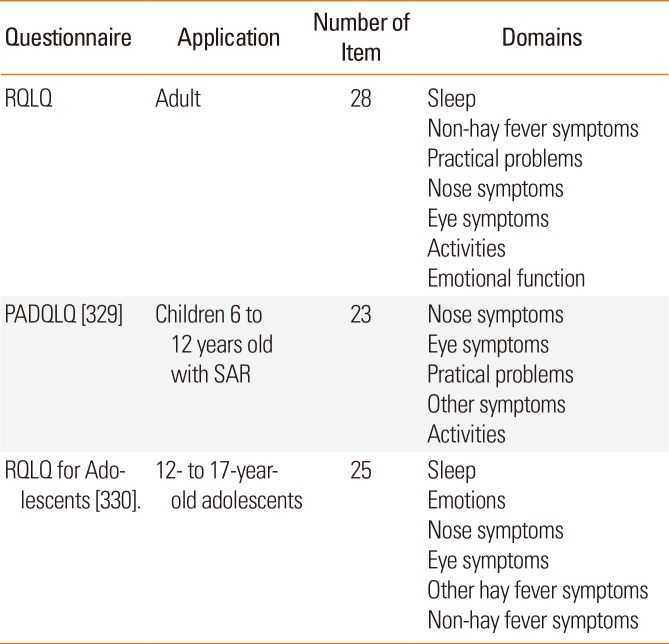
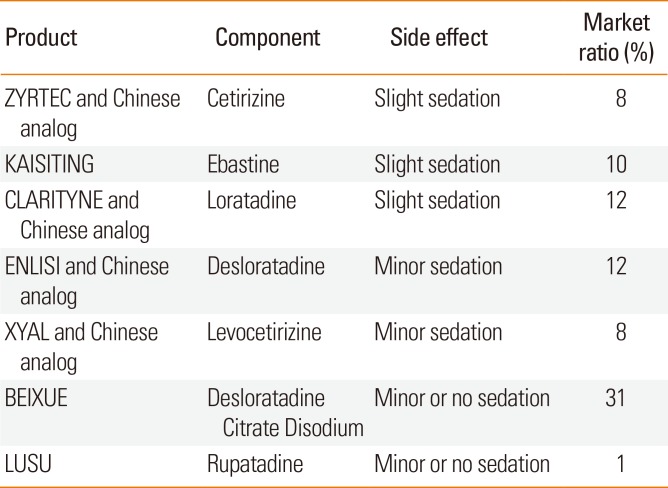
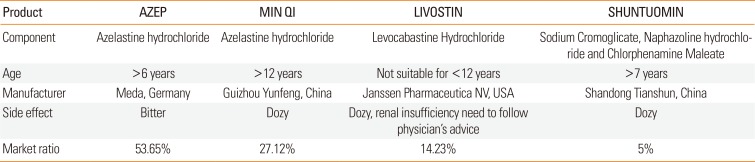
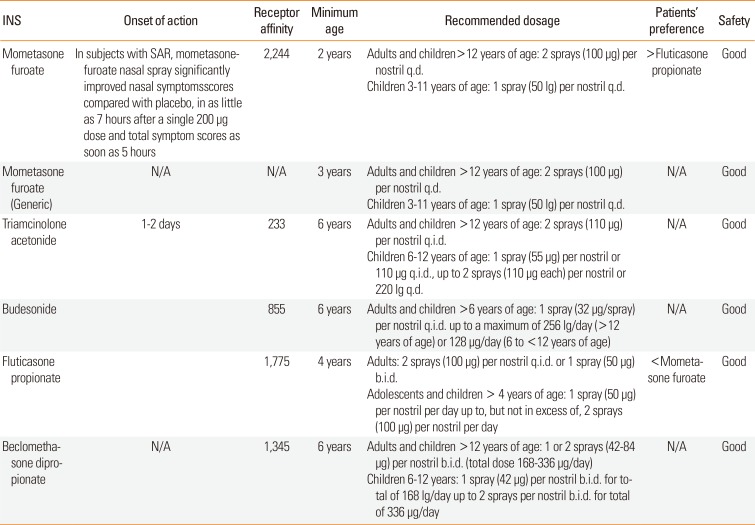

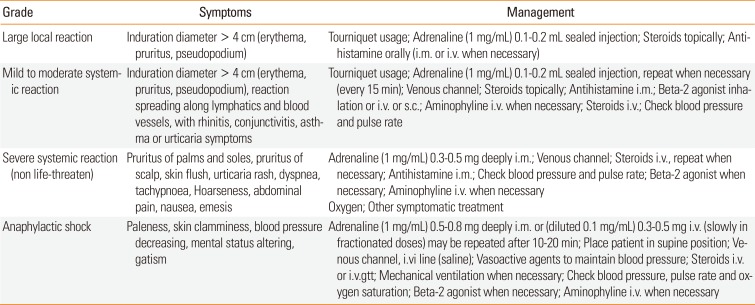
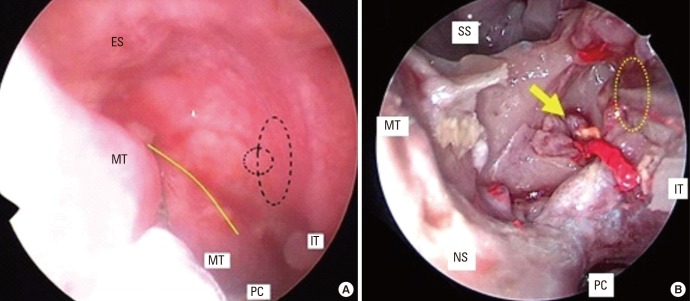




 PDF
PDF ePub
ePub Citation
Citation Print
Print



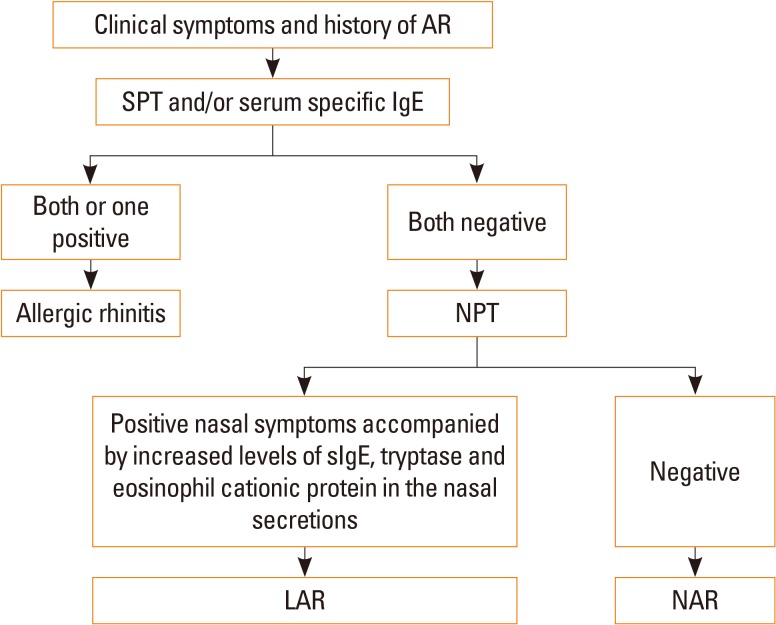
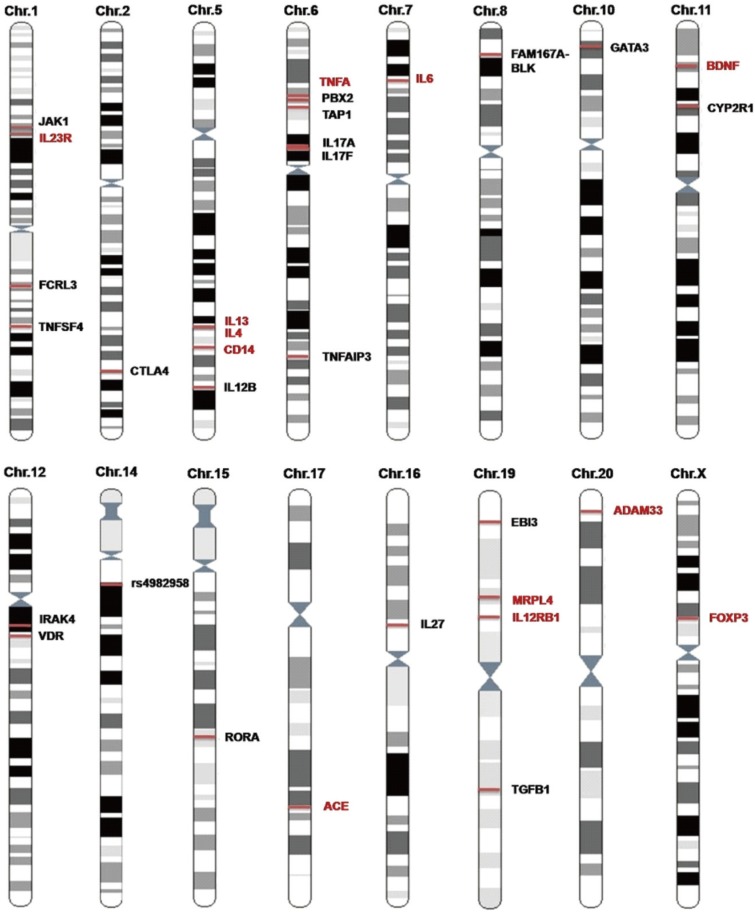
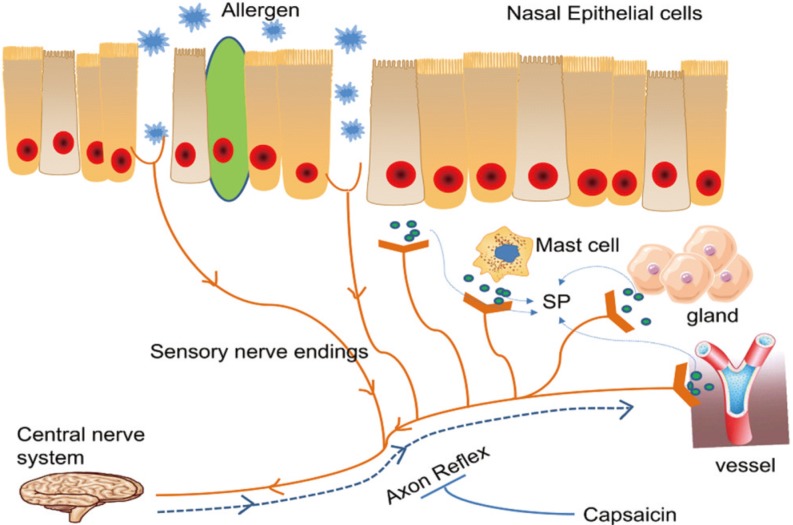
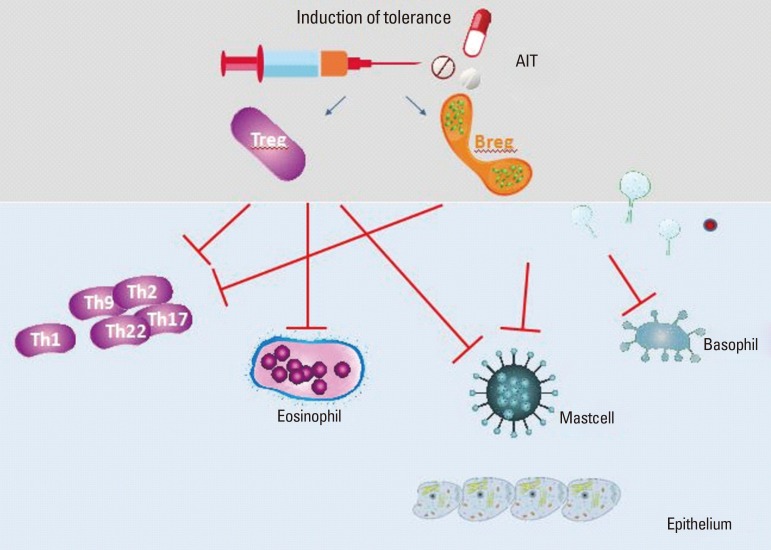
 XML Download
XML Download