Abstract
Spinal tuberculosis (ST) is the tuberculosis caused by Mycobacterium tuberculosis (Mtb) infections in spinal curds. Isoliquiritigenin 4,2′,4′-trihydroxychalcone, ISL) is an anti-inflammatory flavonoid derived from licorice (Glycyrrhiza uralensis), a Chinese traditional medicine. In this study, we evaluated the potential of ISL in treating ST in New Zealand white rabbit models. In the model, rabbits (n=40) were infected with Mtb strain H37Rv or not in their 6th lumbar vertebral bodies. Since the day of infection, rabbits were treated with 20 mg/kg and 100 mg/kg of ISL respectively. After 10 weeks of treatments, the adjacent vertebral bone tissues of rabbits were analyzed through Hematoxylin-Eosin staining. The relative expression of Monocyte chemoattractant protein-1 (MCP-1/CCL2), transcription factor κB (NF-κB) p65 in lymphocytes were verified through reverse transcription quantitative real-time PCR (RT-qPCR), western blotting and enzyme-linked immunosorbent assays (ELISA). The serum level of interleukin (IL)-2, IL-4, IL-10 and interferon γ (IFN-γ) were evaluated through ELISA. The effects of ISL on the phosphorylation of IκBα, IKKα/β and p65 in NF-κB signaling pathways were assessed through western blotting. In the results, ISL has been shown to effectively attenuate the granulation inside adjacent vertebral tissues. The relative level of MCP-1, p65 and IL-4 and IL-10 were retrieved. NF-κB signaling was inhibited, in which the phosphorylation of p65, IκBα and IKKα/β were suppressed whereas the level of IκBα were elevated. In conclusion, ISL might be an effective drug that inhibited the formation of granulomas through downregulating MCP-1, NF-κB, IL-4 and IL-10 in treating ST.
Spinal tuberculosis (ST), also called Pott's disease, is caused by infections of Mycobacterium tuberculosis (Mtb) at spinal cavities [1]. ST usually begins with back pain. The progression of the disease may lead to the spread of abscess into spinal curds, which finally accounts for the spinal collapse, neurologic deficits and even paralysis [123]. As one of the oldest diseases, the history of SP dates back to 3400 BC Egypt [4]. In mid-20th century, SP was once nearly eliminated due to the developments of anti-tuberculosis agents such as isoniazid, rifampicin and streptomycin [5]. However, the prevalence of human acquired immune deficiency syndromes (AIDs) and the development of multi-drug resistance strains in Mtb have led to the recurrence of tuberculosis especially in developing countries [15]. Though multi-drug resistances are not common in SP, the main problems now are focusing on the irreversible bone destructions and neurological deficits caused by chronic inflammation [67]. According to WHO recommendations, different combinations of anti-tuberculosis drugs are selected in treating different subtypes and stages of ST [8]. However, anti-inflammatory therapies were not involved. Hence, it would be important to develop new drugs to relive the chronic inflammation in ST patients.
One hallmark of TB is the formation of granulomas, which are aggregates of a series of well-organized immune cells mainly macrophages and lymphocytes enclosing the stimulus that cannot be absorbed [9]. Granulomas was once been considered a protective structure in eradicating Mtb, however, recent studies have demonstrated that granulomas are important for the dissemination of pathogens [91011]. Mtb can survive for decades inside granulomas, which is one of the major causes of clinical latency and the recurrence of the disease [12].
The formation of granulomas begins with the elevated secretion of chemokines and pro-inflammatory cytokines [13]. Monocyte chemoattractant protein-1 (MCP-1/CCL2) is one of the most studied chemokines of C-C chemokine family. The secretion of MCP-1 is involved in the recruitment and activation of monocytes and T-lymphocytes during the formation of granulomas [14]. Nuclear factor κB (NF-κB) is a multifunctional transcription factor controlling the expression of multiple pro-inflammatory cytokines and chemokines. Hence, it is the central signaling pathway in inflammatory responses. Researches have demonstrated that the polymorphism of MCP-1 determined the susceptibility to TB [15]. Tumor necrosis factor (TNF), the activator of NF-κB, has been shown essential in restricting the growth of bacteria in granulomas [121617]. In previous study, the expression of MCP-1 and NF-κB are both upregulated in New Zealand rabbits carrying ST [18]. Hence, both of the proteins can be important targets in attenuating the inflammatory effects in ST.
Isoliquiritigenin (4,2′,4′-trihydroxychalcone, ISL) is a flavonoid isolated from the roots of licorice (Glycyrrhiza uralensis), which has long been used as a Chinese traditional medicine. The anti-inflammatory effects of ISL have been widely demonstrated in previous researches [192021]. In apolipoprotein E-deficient mice models, 20 mg/kg and 100 mg/kg of ISL have been shown effective in attenuating the expression of MCP-1 and pro-inflammatory cytokines such as IL-6, TNF-α [22]. In another study, both MCP-1 and NF-κB were inhibited by ISL in adipose tissue inflammation [21]. We therefore hypothesized that ISL might be a potential drug in attenuating the granulomas formation through downregulating the expression of MCP-1 and NF-κB. In present study, New Zealand white rabbits were infected with Mtb at spine and were treated with ISL. The relative expression levels of MCP-1 and NF-κB and the serum levels of inflammatory cytokines were evaluated. We demonstrated that ISL might be an effective agent in attenuating the chronic inflammatory symptoms in ST.
H37Rv standard strain was obtained from Fifth Hospital of Harbin, China, and was cultured on Roche culture medium (Jinan Cell Biology, Shandong, China) for two weeks. Colonies with good growth were picked out and dissolved in sterilized normal saline. The final concentration of H37Rv was 1 mg/ml.
ISL (Purity: 98.26%) was purchased from Shaanxi Green Bio-Engineering Co., Ltd. (Xi'an, China), and it was dissolved in 0.5% sodium carboxymethyl cellulose (Sigma Aldrich, St. Louis, MO, USA).
New Zealand white rabbits (3–4 weeks; 3.25±0.25 kg) were randomly divided into 4 groups: infection groups (n=30, including Spinal tuberculosis experimental group, Spinal tuberculosis experimental with 20 mg/kg ISL treated group, Spinal tuberculosis experimental with 100 mg/kg ISL treated group, n=10 in each group) and control group (n=10). All of the rabbits were anesthetized with 2% barbital sodium indodine (20 mg/kg; Sigma Aldrich), and holes were drilled on the 6th lumbar vertebras of rabbits. Gelfoam sponges socked in 0.1 ml H37Rv suspension or normal saline were placed into the perforations of these rabbits. After suturing the incisions, these rabbits were fed alone and 20 mg/kg ISL, 100 mg/kg ISL or normal saline were injected into these rabbits through auricular vein everyday according the groups they belong. The treatment continued for 10 weeks, and tissues surrounding the 5–7 lumbar vertebral bones were dissected for Hematoxylin-Eosin (HE, Sigma Aldrich) staining and peripheral venous bloods were collected for later analyses. All of the experiments were approved by Ethics Committee of Institutional Animal Care and Use Committee at National Defense Medical Center.
Lymphocytes inside the blood from rabbits were isolated through lymphocytes separation medium (Mediatech, Manassas, USA). The isolated lymphocytes were sonicated and centrifuged. Proteins in the supernatants of cell lysates were separated by 12% polyacrylamide gel electrophoresis. After SDS-PAGE, the isolated proteins were transferred onto polyvinylidene fluoride membranes (Sigma). The membranes were then washed twice with cold Tris-buffered saline (TBS) buffer and blocked by 3% BSA. The blocked membranes were then incubated with any of anti-MCP-1, anti-p65, anti-P-p65, anti-IκBα, anti-P-IκBα, anti-IKKα/β, anti-P-IKKα/β or anti-β-actin primary antibodies (Abcam, Cambridge, MA, USA) diluted in the ratio of 1:1,000 for 2 h, in which antibody of β-actin was used as internal control. The primary antibodies were then washed with cold PBS buffer and were again incubated with HRP-conjugated secondary antibody (Abcam) diluted in the ratio of 1: 10,000 for 2 h. The signals on the membrane were then reacted with enhanced chemiluminescence (ECL, Thermo fisher scientific, Waltham, MA, USA) substrate for 1 min at room temperature. The signals on the membrane were detected through charge-coupled device (CCD, Thermo fisher scientific, USA) digital imaging. All of the experiments were triplicated.
To determine the concentration of pro-inflammatory cytokines in serum or the relative expressions of proteins inside lymphocytes, the serums or supernatants of lymphocyte lysates were serial diluted and 3 dilutions were loaded into each well of 96 well plates. Five dilutions of standard samples (50 µL, Shenzhen Jingmei Biological Engineering Co., Ltd., China) of desired proteins were loaded as control. The plates were then washed by TBS buffer, dried and then blocked by 3% BSA. Primary antibodies (100 µL) previously used in western blotting or antibodies of IFN-γ, IL-2, IL-4 and IL-10 (Abcam) were added into plates. After 1 h of incubation, the plates were washed and again incubated with 100 µL HRP conjugate secondary antibodies. The plates were then washed twice with TBS buffer and dried. Chromogenic substrates (100 µL, Thermo fisher scientific) were then added the plates and reacted for 30 min at room temperature in dark. Stop solution (100 µL, Thermo fisher scientific) were then added into each well, and the absorbance detected using Varioskan LUX Multimode Microplate Reader (Thermo fisher scientific) at the wave lengths of 450 nm and 550 nm. The absorbencies were normalized based on the data of standard samples. All of the experiments were repeated three times.
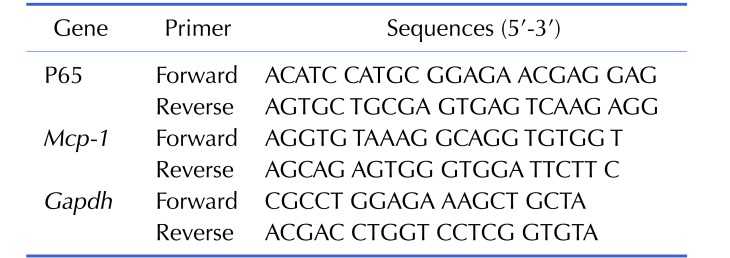
The relative expression of MCP-1 and p65 in lymphocytes were investigated through RT-qPCR. The mRNAs in cell lysates of lymphocytes were reverse transcribed into first line cDNA using Quant Reverse Transcriptase (Tiangen biotech, Beijing, China). The cDNAs then processed quantitative PCR using Fast-Fire qPCR PreMix (Probe; Tiangen) in ABI7500 Real-time PCR (Thermo fisher scientific) system. The expression of β-actin was determined as internal control. Primers were designed for the qRT-PCR (Table 1). The relative expression of these mRNAs was normalized to the expressions in control group. All of the experiments were triplicated.
All of the data were analyzed through SPSS 22.0 system (IBM, Armonk, NY, USA), and presented as mean±standard deviation (SD). The differences between two groups of data were analyzed through student T tests and single factor variance analysis (ANOVA). Chi-square test was used to analyze data shown in percentage. p<0.05 can be considered statistical significant.
To understand the effects of isoliquiritigenin in relieving the inflammatory symptoms in SP, 40 rabbits were divided into four groups. Among the 40 rabbits, 30 of them were infected with H37Rv cells from M. tuberculosis strain, and another 10 rabbits without infection were leaved as control group. Since the day of infection, 20 mg/kg, 100 mg/kg of ISL or placebo were injected to rabbits everyday through auricular vein according to the experimental groups they belonged. After 10 week of treatments, all of the rabbits were anesthetized and tissues from adjacent vertebral bone tissues were dissected, HE stained and analyzed. Comparing to the specimens from the control group, tissues from the rabbits with ST were darker and contained plenty of granulomatous, in which caseous necrosis was surrounded by Langhans giant cells (Figs. 1A and B). The treatment of 20 mg/kg ISL was effective enough to decrease the formation of granulomatous, whereas 100 mg/kg of ISL retrieve the tissues to the state similar to the features in healthy tissues (Figs. 1C and D).
One way to present the degree of inflammation is to analyze the expression of biomarkers of inflammation, which in our experiments were MCP-1 and NF-κB. We firstly investigated their relative expressions of mRNAs using RT-qPCR. The expressions of both proteins were raised in ST groups, and were inhibited under the treatments of ISL (Fig. 2A, F=37.55, p<0.05; F=101.33, p<0.05, respectively for MCP-1 and p65). We then analyzed the expression of MCP-1 and NF-κB at protein level through western blotting and ELISA (Figs. 2B and C; F=43.78, p<0.05; F=62.11, p<0.05, respectively for MCP-1 and p65). The effects in decreasing the expression of MCP-1 and p65 were not significant in 20 mg/kg ISL treated group. However, its efficacy increased markedly when the dosage of ISL raised to 100 mg/kg (Figs. 2B and D; F=17.94, p<0.05; F=52.73, p<0.05, respectively for MCP-1 and p65). It was obvious that the expressions of both proteins were relatively low (around 200 pg/ml) in healthy tissues, and the Mtb infection raised their expression to almost 2 to 3 times when compared with the original protein level in infected groups (Fig. 2D, p<0.05).
Pro-inflammatory cytokines are important proteins involved in innate and acquired immune responses. We examined the serum level of pro-inflammatory cytokines interferon γ (IFN-γ), interleukin-2 (IL-2), IL-4 and IL-10 by ELISA. In general, Mtb infection induced the secretion of all of these cytokines except IL-2 in experimental groups in comparison with them of control group (Fig. 3, all p<0.05, F=58.19, F=9.67, F=26.34 and F=42.13, respectively for IFN-γ, IL-2, IL-4 and IL-10). The treatment of 20 mg/kg of ISL slightly inhibited the serum level of IL-4 and IL-10 when compared with the ST rabbits, However, they were mostly suppressed in ST rabbits treated with 100 mg/kg of ISL. The function of ISL on IFN-γ and IL-2 cannot be determined since little differences can be discovered when comparing their expression in ISL groups with control group or ST group.
We then investigated the role of NF-κB signaling pathways in the treatment of ISL. IκBα is an important biomarker involved in the inhibition of NF-κB transcription factors. The existence of IκBα and P-IκBα were evaluated through western blotting. The concentrations of IκBα were significantly decreased in the ST group, whereas the concentrations of P-IκBα were increased (Fig. 4A). The effects of 20 mg/kg of ISL were not significant enough to be shown on the results of western blot of both proteins. However, the treatments of 100 mg/kg of ISL markedly recovered the expressions of IκBα and decreased the concentrations of P-IκBα to the level similar to healthy tissues. The relative expressions of IκBα and P-IκBα were further quantified by ELISA, which were consistent with the results from western blotting. The treatment of 100 mg/kg of ISL was effective enough to reverse the expression of IκBα and P-IκBα towards the levels of the control group (Fig. 4B, F=17.27 p<0.05; Fig. 4C, F=50.04, p<0.05). The functions of 20 mf/kg ISL were not significant when comparing with the ST group (p<0.05).
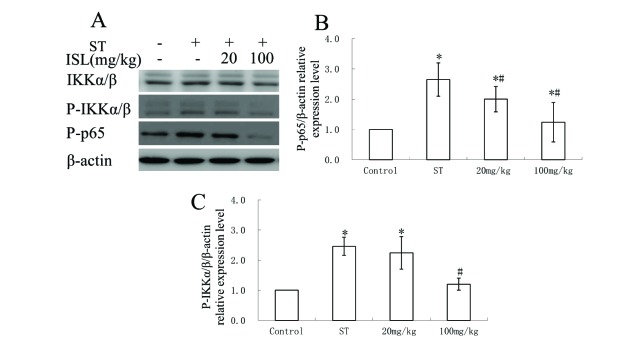
We then verified other modulators involved in NF-κB signaling pathways including IKKα/β, P-IKKα/β and P-p65 inside adjuvant vertebral tissues through western blotting and ELISA (Fig. 5). From the results of western blotting, the existences of IKKα/β were not influenced significantly in different groups of treatments (Fig. 5A). The phosphorylation levels of IKKα/β and p65 were increased in ST group and 20 mg/kg ISL treated groups, and were decreased to concentrations similar to healthy rabbits in 100 mg/ml ISL treated group. The relative expression of P-IKKα/β and P-p65 were further verified through ELISA analysis, in which the expression of both of the proteins were elevated more than two times after the ST infection (Fig. 5B, F=32.23, p<0.05; Fig. 5C, F=75.77, p<0.05). The treatment of 20 mg/kg of ISL decreased the concentration of P-p65, however, its effect in regulating P-IKKα/β was not significant (p<0.05). In 100 mg/kg group, the expression of both of the proteins were markedly inhibited, and even the differences between the concentrations of P-IKKα/β in control group and 100 mg/kg group was not significant (p<0.05).
ST is the most occurred non-pulmonary tuberculosis in China, which account for more than 50% of total cases [18]. The progression of disease could finally lead to spinal deformity, neural dissects and even paraplegia, which are irreversible damages not directly caused by the Mtb infection but prolonged inflammatory responses surrounding the infected tissues [1]. Researches have demonstrated the antimicrobial potential of ISL and its analogues in H37Rv, H37Ra and RV0636 Mtb strains [232425]. The antiinflammatory effects of ISL has been shown in apolipoprotein E-deficient mouse models, 20 mg/kg and 100 mg/kg of which were effective in attenuating Atherogenesis [22]. In this research, we meant to investigate the efficacy of ISL in treating tuberculosis in vivo. In most cases mouse models are effective enough in studying human disease, however, granulomas with caseous necrosis are rarely formed in TB rats [26]. Hence, mouth models are rarely employed in ST researches. On the contrary, the pathology of TB in rabbits were similar to human symptoms, in which latent TB can be reactivated when immune balances are broken [26]. Thereby, the New Zealand white rabbits were employed in evaluating the function of ISL in the dosage of 20 mg/kg and 100 mg/kg in treating ST.
Granulomas are characterized by the accumulation of differentially differentiated macrophages, including Langhans giant cells, epithelioid cells and foam cells [313]. The formation of multinucleated Lanhans giant cells through the infusion of multiple macrophages is special to the granulomas formed in TB patients [3]. The continuous aggregation of immune cells leads to the formation caseous necrosis in the center of granulomas and subsequently liquefaction of the necrosis tissues, which finally lead to irreversible damages [27]. Hence it is crucial to inhibit the formation of granulomas in treating ST. In our results the continuous treatments of 100 mg/kg ISL were effective enough in suppressing the formation of granulomas. Experiments have shown that ISL inhibit carmine contents inside granulomas through its anti-tube formation effects in 1995 [28]. The anti-angiogenesis effects of ISL have also been demonstrated in breast cancer cells and ocular angiogenesis models [2930]. However, studies investigating the mechanisms of the anti-angiogenesis effects of ISL are still limited.
The first step to the granulation is the elevated secretion of proinflammatory cytokines and chemokines at the site of infection [10]. MCP-1 is one of the most basic chemokines, the deletion of which could delay the monocyte recruitments for 72 h [31]. Also, the polymorphism of MCP-1 has been shown influenced the susceptibities of patients to Mtb to up to 6.8 times through controlling the expression of IL-12p40 [1532]. In our previous study, the relative expression of MCP-1 was upregulated after Mtb infection in New Zealand rabbit models [17]. Hence, MCP-1 can be a potent target in treating ST. ISL has been shown to inhibit the mRNA expression of MCP-1 in macrophages in atherogenesis and in adipose tissue inflammation [2122]. In present research, we further demonstrated that 100 mg/kg of ISL injections were effective enough to suppress the expression of MCP-1 to the level similar to healthy rabbits.
In previous research, the expression of NF-κB subtype p65 has been shown elevated in lymphocytes in New Zealand white rabbits with ST [18]. NF-κB is potent in inflammatory responses in most diseases, however, the overexpression of it might be deleterious. According to the result of a mathematical modeling among the dosage of NF-κB inside cells, sufficient amount of NF-κB is efficient to kill bacteria, whereas the higher level of it might lead to the aggregation of higher amount of uninfected macrophages, which subsequently promote granulation [33]. In another study, the inhibition of NF-κB even suppressed the Mtb survival in human macrophages through promoting apoptosis and autophagy of infected cells [34]. ISL has been shown anti-osteoclastogenic functions through suppressing NF-κB expression and translocation in both RAW 264.7 macrophages and in mouth models [35]. In another experiment, p65 was notably inhibited by ISL in LPS induced macrophages [36]. In our results, the Mtb induced p65 expressions were markedly inhibited by 100 mg/kg of ISL. Hence, ISL probably inhibited the excessive inflammation during granulation.
We further demonstrated that ISL suppressed the signaling of NF-Kb in ST rabbits, in which the relative expression of the IκBα were downregulated, whereas the phosphorylation of its upstream regulator IKKα/β were elevated. The effects of ISL to NF-κB signaling have been reported in RAW264.7 macrophages and psoriasis mouse models [2037]. The function of ISL in suppressing the expression of NF-κB is not strange. Actually, it has been demonstrated inhibiting IKK through binding to the cysteine 46 of the protein [38].
ISL has been shown inhibiting NF-κB and its downstream proinflammatory cytokines such as IL-6 and IL-8 in various psoriasis models [37]. In this research, the serum level of IL-4 and IL-10 were significantly suppressed by ISL, however, its function to IL-2 and IFN-γ was limited. IL-4 is involved in the activation of type 2 T helper (Th2) cells. In mouse models, IL-4 has been shown highly expressed during the progressive phase of TB [39]. However, the researches among the regulatory effects of ISL to IL-4 are still limited. IL-10, also called inhibitors of cytokine synthesis, is capable of inhibiting the activation of Th1 cells [40]. In pulmonary tuberculosis, IL-10 is upregulated and the neutralization of which could promoted the proliferation of T lymphocytes [41]. The inhibition of IL-10 by ISL has been demonstrated in M2 macrophages during the development of colorectal cancer [42]. IFN-γ is an important pro-inflammatory cytokine involved in the activation of macrophages in TB In previous experiments, the higher level of INF-γ is related to the poor outcomes in ST patients [43]. IL-2 is another cytokine promoting the activation, proliferation and differentiation most immune cells in response to antigenic activation [44]. All of the four cytokines are controlled by the NF-κB transcription factor. However, the treatment of ISL to the serum level of IFN-γ and IL-2 were not significant enough. In psoriasis models the treatment of ISL also did not influenced the serum level of IL-2 [37]. Hence further experiments are necessary in understanding how the expression of cytokines are influenced by the treatment of ISL in treating ST.
In conclusion, in this research we investigated the potential of Isoliquiritigenin as a novel drug in treating spinal tuberculosis. We demonstrated that 100 mg/kg of LSP was effetive enough in supressing the formation of granulomas, which is probably caused by inhibiting the expression of MCP-1 and NF-kB, and the inhibition of IL-10 and IL-4 anti-inflammatory cytokines.
Notes
References
1. Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med. 2011; 34:440–454. PMID: 22118251.

2. Kumar K. Spinal tuberculosis, natural history of disease, classifications and principles of management with historical perspective. Eur J Orthop Surg Traumatol. 2016; 26:551–558. PMID: 27435619.

3. Danaviah S, Sacks JA, Kumar KP, Taylor LM, Fallows DA, Naicker T, Ndung'u T, Govender S, Kaplan G. Immunohistological characterization of spinal TB granulomas from HIV-negative and -positive patients. Tuberculosis (Edinb). 2013; 93:432–441. PMID: 23541388.

4. Taylor GM, Murphy E, Hopkins R, Rutland P, Chistov Y. First report of Mycobacterium bovis DNA in human remains from the Iron Age. Microbiology. 2007; 153:1243–1249. PMID: 17379733.

5. Rajasekaran S, Khandelwal G. Drug therapy in spinal tuberculosis. Eur Spine J. 2013; 22(Suppl 4):587–593. PMID: 22581190.

6. Pawar UM, Kundnani V, Agashe V, Nene A, Nene A. Multidrug-resistant tuberculosis of the spine−is it the beginning of the end? A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine (Phila Pa 1976). 2009; 34:E806–E810. PMID: 19829244.
7. Hoshino A, Hanada S, Yamada H, Mii S, Takahashi M, Mitarai S, Yamamoto K, Manome Y. Mycobacterium tuberculosis escapes from the phagosomes of infected human osteoclasts reprograms osteoclast development via dysregulation of cytokines and chemokines. Pathog Dis. 2014; 70:28–39. PMID: 23929604.
8. World Health Organization. Stop TB Initiative. Treatment of tuberculosis: guidelines. 4th ed. Geneva: World Health Organization;2010.
9. Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012; 12:352–366. PMID: 22517424.

10. Bold TD, Ernst JD. Who benefits from granulomas, mycobacteria or host? Cell. 2009; 136:17–19. PMID: 19135882.

11. Rubin EJ. The granuloma in tuberculosis−friend or foe? N Engl J Med. 2009; 360:2471–2473. PMID: 19494225.
12. Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol. 2010; 185:15–22. PMID: 20562268.

13. Guirado E, Schlesinger LS. Modeling the Mycobacterium tuberculosis granuloma - the critical battlefield in host immunity and disease. Front Immunol. 2013; 4:98. PMID: 23626591.

14. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994; 91:3652–3656. PMID: 8170963.

15. Mecabih F, Sadouki F, Bennabi M, Salah S, Boukouaci W, Amroun H, Fortier C, Marzais F, Charron D, Djoudi H, Abbadi MC, Krishnamoorthy R, Tamouza R. Functional polymorphisms of Monocyte Chemoattractant Protein-1 gene and Pott's disease risk. Immunobiology. 2016; 221:462–467. PMID: 26626202.

16. Fallahi-Sichani M, El-Kebir M, Marino S, Kirschner DE, Linderman JJ. Multiscale computational modeling reveals a critical role for TNF-α receptor 1 dynamics in tuberculosis granuloma formation. J Immunol. 2011; 186:3472–3483. PMID: 21321109.

17. Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008; 29:283–294. PMID: 18691913.

18. Guo XH, Bai Z, Qiang B, Bu FH, Zhao N. Roles of monocyte chemotactic protein 1 and nuclear factor-κB in immune response to spinal tuberculosis in a New Zealand white rabbit model. Braz J Med Biol Res. 2017; 50:e5625. PMID: 28225889.

19. Kwon HM, Choi YJ, Choi JS, Kang SW, Bae JY, Kang IJ, Jun JG, Lee SS, Lim SS, Kang YH. Blockade of cytokine-induced endothelial cell adhesion molecule expression by licorice isoliquiritigenin through NF-kappaB signal disruption. Exp Biol Med (Maywood). 2007; 232:235–245. PMID: 17259331.
20. Kim JY, Park SJ, Yun KJ, Cho YW, Park HJ, Lee KT. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages. Eur J Pharmacol. 2008; 584:175–184. PMID: 18295200.
21. Watanabe Y, Nagai Y, Honda H, Okamoto N, Yamamoto S, Hamashima T, Ishii Y, Tanaka M, Suganami T, Sasahara M, Miyake K, Takatsu K. Isoliquiritigenin attenuates adipose tissue inflammation in vitro and adipose tissue fibrosis through inhibition of innate immune responses in mice. Sci Rep. 2016; 6:23097. PMID: 26975571.

22. Du F, Gesang Q, Cao J, Qian M, Ma L, Wu D, Yu H. Isoliquiritigenin attenuates atherogenesis in apolipoprotein E-deficient mice. Int J Mol Sci. 2016; 17.

23. Chokchaisiri R, Suaisom C, Sriphota S, Chindaduang A, Chuprajob T, Suksamrarn A. Bioactive flavonoids of the flowers of Butea monosperma. Chem Pharm Bull (Tokyo). 2009; 57:428–432. PMID: 19336944.

24. Brown AK, Papaemmanouil A, Bhowruth V, Bhatt A, Dover LG, Besra GS. Flavonoid inhibitors as novel antimycobacterial agents targeting Rv0636, a putative dehydratase enzyme involved in Mycobacterium tuberculosis fatty acid synthase II. Microbiology. 2007; 153:3314–3322. PMID: 17906131.
25. Gaur R, Thakur JP, Yadav DK, Kapkoti DS, Verma RK, Gupta N, Khan F, Saikia D, Bhakuni RS. Erratum to: Synthesis, antitubercular activity, and molecular modeling studies of analogues of isoliquiritigenin and liquiritigenin, bioactive components from Glycyrrhiza glabra. Med Chem Res. 2015; 24:3772–3774.
26. Barry CE 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009; 7:845–855. PMID: 19855401.

27. Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, Tsenova L, Kaplan G, Russell DG. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010; 2:258–274. PMID: 20597103.

28. Kobayashi S, Miyamoto T, Kimura I, Kimura M. Inhibitory effect of isoliquiritin, a compound in licorice root, on angiogenesis in vivo and tube formation in vitro. Biol Pharm Bull. 1995; 18:1382–1386. PMID: 8593441.

29. Wang Z, Wang N, Han S, Wang D, Mo S, Yu L, Huang H, Tsui K, Shen J, Chen J. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS One. 2013; 8:e68566. PMID: 23861918.

30. Jhanji V, Liu H, Law K, Lee VY, Huang SF, Pang CP, Yam GH. Isoliquiritigenin from licorice root suppressed neovascularisation in experimental ocular angiogenesis models. Br J Ophthalmol. 2011; 95:1309–1315. PMID: 21719569.

31. Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998; 187:601–608. PMID: 9463410.

32. Flores-Villanueva PO, Ruiz-Morales JA, Song CH, Flores LM, Jo EK, Montaño M, Barnes PF, Selman M, Granados J. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J Exp Med. 2005; 202:1649–1658. PMID: 16352737.

33. Fallahi-Sichani M, Kirschner DE, Linderman JJ. NF-κB signaling dynamics play a key role in infection control in tuberculosis. Front Physiol. 2012; 3:170. PMID: 22685435.

34. Bai X, Feldman NE, Chmura K, Ovrutsky AR, Su WL, Griffin L, Pyeon D, McGibney MT, Strand MJ, Numata M, Murakami S, Gaido L, Honda JR, Kinney WH, Oberley-Deegan RE, Voelker DR, Ordway DJ, Chan ED. Inhibition of nuclear factor-kappa B activation decreases survival of Mycobacterium tuberculosis in human macrophages. PLoS One. 2013; 8:e61925. PMID: 23634218.

35. Liu S, Zhu L, Zhang J, Yu J, Cheng X, Peng B. Anti-osteoclastogenic activity of isoliquiritigenin via inhibition of NF-κB-dependent autophagic pathway. Biochem Pharmacol. 2016; 106:82–93. PMID: 26947453.

36. Feldman M, Santos J, Grenier D. Comparative evaluation of two structurally related flavonoids, isoliquiritigenin and liquiritigenin, for their oral infection therapeutic potential. J Nat Prod. 2011; 74:1862–1867. PMID: 21866899.

37. Wu Y, Chen X, Ge X, Xia H, Wang Y, Su S, Li W, Yang T, Wei M, Zhang H, Gou L, Li J, Jiang X, Yang J. Isoliquiritigenin prevents the progression of psoriasis-like symptoms by inhibiting NF-κB and proinflammatory cytokines. J Mol Med (Berl). 2016; 94:195–206. PMID: 26383911.

38. Yan F, Yang F, Wang R, Yao XJ, Bai L, Zeng X, Huang J, Wong VKW, Lam CWK, Zhou H, Su X, Liu J, Li T, Liu L. Isoliquiritigenin suppresses human T lymphocyte activation via covalently binding cysteine 46 of IκB kinase. Oncotarget. 2017; 8:34223–34235. PMID: 27626700.

39. Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004; 25:483–488. PMID: 15324741.

40. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001; 19:683–765. PMID: 11244051.

41. Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994; 94:2435–2442. PMID: 7989601.

42. Zhao H, Zhang X, Chen X, Li Y, Ke Z, Tang T, Chai H, Guo AM, Chen H, Yang J. Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE2 and IL-6. Toxicol Appl Pharmacol. 2014; 279:311–321. PMID: 25026504.

43. Patil T, Garg RK, Jain A, Goel MM, Malhotra HS, Verma R, Singh GP, Sharma PK. Serum and CSF cytokines and matrix metalloproteinases in spinal tuberculosis. Inflamm Res. 2015; 64:97–106. PMID: 25503789.

44. Goyal N, Kashyap B, Kaur IR. Significance of IFN-γ/IL-2 ratio as a circulating diagnostic biomarker in extrapulmonary tuberculosis. Scand J Immunol. 2016; 83:338–344. PMID: 26946082.

Fig. 1
Hematoxylin-eosin staining of adjacent vertebral bone tissues of rabbits.
(A) Sham operated control group. (B) Spinal tuberculosis experimental group. (C) Spinal tuberculosis experimental with 20 mg/kg Isoliquiritigenin treatment. (D) Spinal tuberculosis experimental with 100 mg/kg Isoliquiritigenin treatment. Arrow: the granulomatous inflammation with Langhans giant cells surrounding the caseous necrosis.
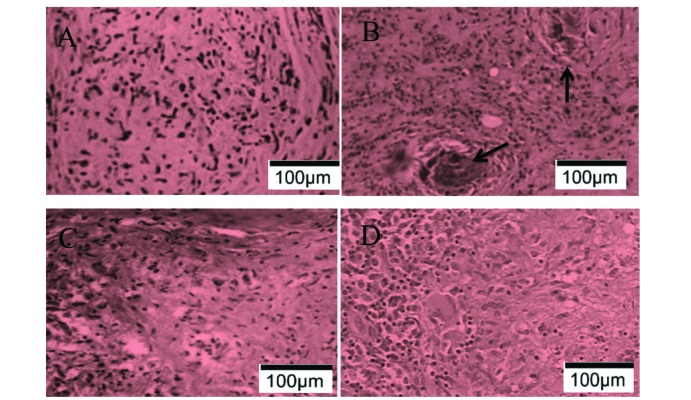
Fig. 2
The effect of ISL on the expression of monocyte chemotactic protein 1 (MCP-1) and nuclear factor kappa B (NF-κB) in lymphocytes.
(A) Relative mRNA expression of monocyte chemotactic protein 1 (MCP-1) and nuclear factor kappa B (NF-κB), (B) protein expressions of MCP-1 and NF-κB detected by ELISA, (C) Western blotting of the protein expressions of MCP-1 and NF-κB, (D) Protein expressions of MCP-1 and NF-κB. *p<0.05 compared to the sham control groups, #p<0.05 compared to spinal tuberculosis experimental group (n=10). Control, sham operated control group; ST, Spinal tuberculosis experimental group; 20 mg/kg, Spinal tuberculosis experimental with 20 mg/kg ISL treatment; 100 mg/kg, Spinal tuberculosis experimental with 100 mg/kg ISL treatment.
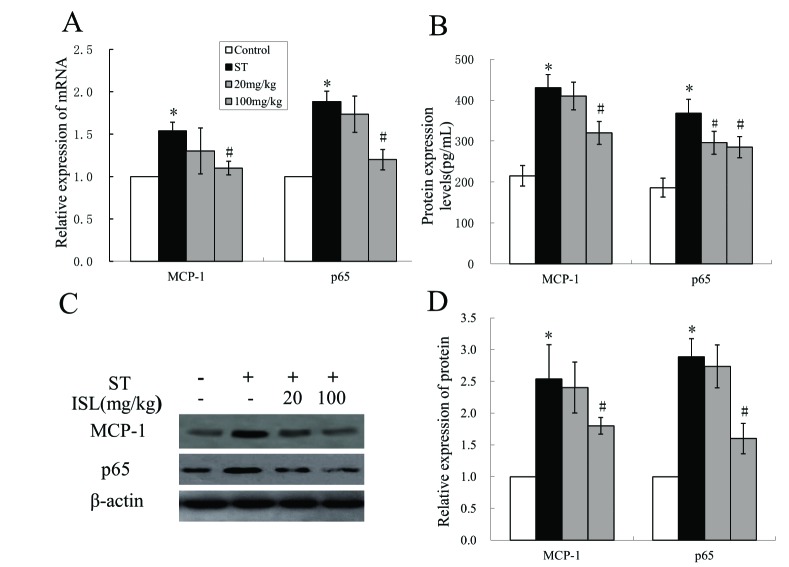
Fig. 3
The effect of ISL on serum levels of IFN-γ, IL-2, IL-4 and IL-10 detected by ELISA.
*p<0.05 compared to the sham control groups, #p<0.05 compared to spinal tuberculosis experimental group (n=10). Control, sham operated control group; ST, Spinal tuberculosis experimental group; 20 mg/kg, Spinal tuberculosis experimental with 20 mg/kg ISL treatment; 100 mg/kg, Spinal tuberculosis experimental with 100 mg/kg ISL treatment.
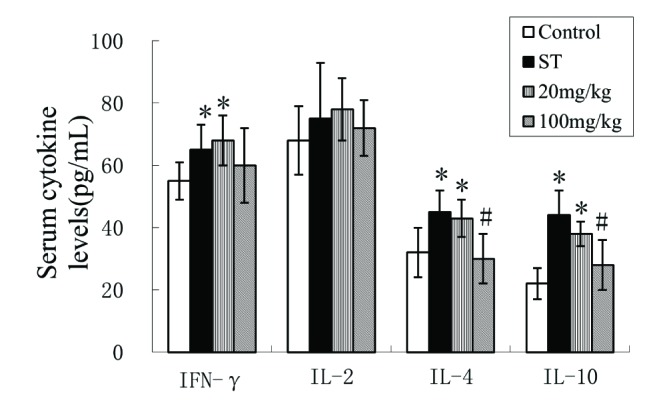
Fig. 4
The effect of ILG on degradation and phosphorylation of IκBα.
(A) Western blotting of the protein expressions of IκBα and P-IκBα in lymphocytes, (B) IκBα/β-actin relative expression level, (C) P-IκBα/β-actin relative expression level. *p<0.05 compared to the sham control groups, #p<0.05 compared to spinal tuberculosis experimental group (n=10). Control: sham operated control group, ST: Spinal tuberculosis experimental group, 20 mg/kg: Spinal tuberculosis experimental with 20 mg/kg ISL treatment, 100 mg/kg: Spinal tuberculosis experimental with 100 mg/kg ISL treatment.
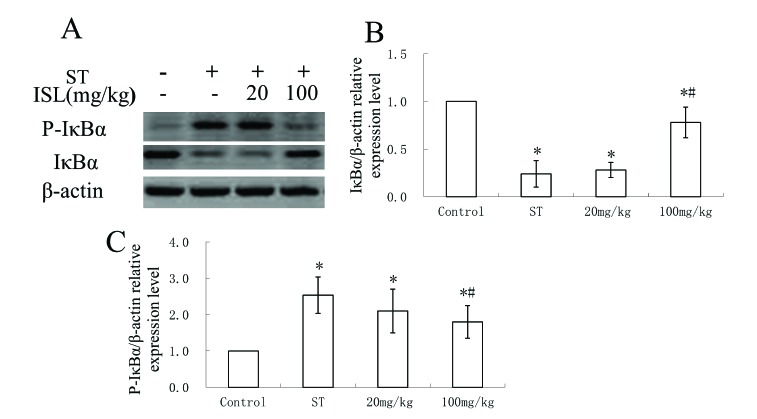
Fig. 5
The effect of ISL on phosphorylation of the nuclear translocation of NF-κB subunit p65 and IKKα/β in lymphocytes.
(A) phosphorylation of the protein expressions of p65 and IKKα/β detected by western blotting, (B) P-p65/β-actin relative expression level, (C) IKKα/β/β-actin relative expression level. *p<0.05 compared to the sham control groups, #p<0.05 compared to spinal tuberculosis experimental group (n=10). Control, Sham operated control group; ST, Spinal tuberculosis experimental group; 20 mg/kg, Spinal tuberculosis experimental with 20 mg/kg ISL treatment; 100 mg/kg, Spinal tuberculosis experimental with 100 mg/kg ISL treatment.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download