INTRODUCTION
Pulmonary hypertension (PH) is a hemodynamic and pathophysiologic condition, defined as an increase in pulmonary artery pressure (PAP) that results in right ventricular (RV) pressure overload and, ultimately, right heart failure and death.
1)
Transthoracic echocardiography provides direct and indirect signs of elevated PAP and can be used as an excellent noninvasive screening test for patients with symptoms or risk factors for PH.
1)2)
D-shaped left ventricle (D-LV), is an interesting echocardiographic finding in PH and is the result of structural distortion of the interventricular septum caused by an abnormal pressure gradient between the left and right ventricles. Although the presence of a D-LV is not a diagnostic feature of RV overload, this finding should prompt further investigation of the etiology and severity of right-sided pressure and volume overload.
3)
The eccentricity index (EI) is a previously described quantitative measure that is used to evaluate septal flattening in patients with PH. The EI is a ratio of the left ventricular (LV) anteroposterior dimension to the septolateral dimension in the parasternal short-axis window. An index greater than 1.0 is abnormal and suggests RV overload.
3)4)
The relationship between D-LV and hemodynamic factors such as RV systolic pressure (RVSP) or LV filling pressure, represented by E/E′, and other echocardiographic indices has not been clearly determined. These factors are important in clinical decision making, therefore, we investigated the echocardiographic parameters related to D-LV and the relationship between EIs and echocardiographic parameters in patients with increased PAP.
METHODS
Study population
We retrospectively analyzed a total of 526 patients with a maximal tricuspid regurgitation velocity (VmaxTR) > 2.8 m/s on echocardiography who were hospitalized at Hanyang University Medical Center between January 2012 and December 2017. For patients with multiple echocardiographic data, only the data initially obtained during the study period was used.
Because of the complex nature of PH, exclusion criteria were applied to homogenize the hemodynamic characteristics of PH, such as severe valve disease or valve replacement status, congenital heart disease, atrial fibrillation or sick sinus syndrome, tachycardia > 120 bpm, large pericardial effusion, leukemia or other cancer, acute shock state, heart failure with reduced ejection fraction of ischemic or non-ischemic origin, stage 4 or 5 chronic kidney disease, severe kyphoscoliosis, and poor windows (n = 237).
Data collection
Demographic and clinical data were collected, including age, sex, body mass index (BMI), blood pressure (BP), and cardiovascular risk factors. Laboratory data, including hemoglobin, B-type natriuretic peptide, and troponin-I levels and estimated glomerular filtration rate were also collected.
Routine echocardiographic examination using a standard commercial echocardiography machine (iE33, Philips) was performed. Echocardiographic parameters, including LV dimension, transmitral flow, E/E′, pulmonary outflow acceleration time, VmaxTR, right atrial pressure (RAP) defined by inferior vena cava size and collapsibility,
1) RVSP defined as 4 × VmaxTR
2 + RAP, and tricuspid annular plane systolic excursion (TAPSE) were obtained. Heart rate (HR) during echocardiography was recorded. LV posterior wall thickness (LVPWT) and interventricular septal thickness (IVST) were measured in end-diastole. End-diastole was defined as the frame in which LV volume is the largest.
Visual assessment confirmed the presence of a D-LV was analyzed in the standard parasternal short-axis view at the level of mid-papillary muscles in end-systole. The EI was obtained from the same frame images. End-systole was defined as the frame in which the smallest short-axis area was obtained. Measurements were performed by a single echocardiography physician.
Statistical analyses
Continuous variables were reported as the mean values with standard deviations and categorical variables were presented as the numbers and percentages. The t-test was used to compare continuous variables between groups, and the chi-squared test was used for categorical variables.
A multivariable logistic regression analysis was performed to evaluate factors that were associated with D-LV in patients with increased PAP that were assessed by echocardiography. Independent variables included HR, PAP, TAPSE, RVSP/systolic BP, LVPWT, and E/E′ in Model 1. The same independent variables along with age and BMI were used in Model 2. For patients with D-LV, factors related to the severity of D-LV defined by EIs were explored by multiple linear regression analysis with the same independent variables.
For all assessments, a p-value < 0.05 indicated a statistically significant result. Statistical analysis was performed using PASW 18.0 (SPSS, Chicago, IL, USA).
DISCUSSION
Our data showed that PAP, TAPSE, E/E′, and age were associated with the presence of D-LV in patients with increased PAP that was assessed by echocardiography. Furthermore, for patients with D-LV, the only factor that related to the severity of D-LV, as defined by EIs, was E/E′. To the best of our knowledge, this is the first study that has investigated factors that are associated with the presence and severity of D-LV in patients with increased PAP that was assessed by echocardiography. Our findings suggest a possible link between E/E′ and D-LV severity in patients with increased PAP.
The EI is a simple measure of septal curvature that was first described by Ryan et al.
5) The study showed that patients with RV pressure overload had systolic septal flattening, whereas patients with RV volume overload had diastolic septal flattening.
5) There have been several previous studies on the determinants of the EIs. Jessup et al.
6) demonstrated that the transseptal pressure gradient is a key determinant of abnormal septal motion and flattening in patients with PH. More recently, Haddad et al.
7) reported a correlation between EIs and ventricular interdependence, and Watson et al.
4) reported that EIs correlated well with RVSP/systolic BP. López-Candales
8) reported that elevated systolic PAP was the best determinant of an abnormal septal curvature in chronic PH. Consistent with a previous study, we have also found that PAP was independently associated with D-LV. However, the interventricular pressure gradient was not significantly associated with D-LV on multivariable analysis, whereas E/E′ was strongly associated with EIs in patients who already presented with D-LV.
Previously, Mahmud et al.
9) reported that E/A was consistently decreased in patients with chronic thromboembolic pulmonary hypertension and that E/A varies inversely with PAP. After pulmonary thromboendarterectomy, E/A and pulmonary capillary wedge pressure were significantly increased in conjunction with decreased PAP. The authors explained that impaired LV filling resulted in distortion of LV cavity geometry caused by RV pressure overload and leftward displacement of the interventricular septum.
9) Our results were related to results of a previous study, which reported that factors associated with D-LV in PH were associated with increased PAP (RV pressure overload) and decreased E/E′ (decreased LV filling). However, it was shown that the severity of D-LV, defined as the EIs, was associated with E/E′ independently of PAP. Based on these results, D-LV might result from a combination of RV pressure overload and distortion of LV cavity geometry, which results in impairment of LV filling, and decreased LV filling aggravates D-LV severity.
Various factors may influence the presence and severity of D-LV. For example, excess weight on LV diastolic dysfunction
10) and RV dysfunction
11) have been documented. Moreover, obesity was associated with decreased functional capacity in patients with PH.
12) As cardiac and pulmonary diseases increase with aging, the prevalence of PH also increases. Previous data have shown that, although PH was once thought to affect young adults, the mean age of patients with PH now represents a growing proportion of older patients.
13) In our study population, there was no difference in BMI between groups, and patients with D-LV were younger than other patients. Although age and BMI may affect PAP, D-LV was not simply determined by PAP but may be determined by interactions between LV and RV hemodynamics.
A similar mechanism is found in the pathophysiology of PTE in that thromboembolism leads to increased pulmonary vascular resistance, resulting in RV pressure overload. Decreased RV output and displacement of the interventricular septum could limit diastolic LV filling.
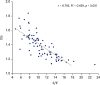
9)14) However, in our study, the association between EIs and E/E′ in patients with D-LV was unchanged when analyzed, except in patients with PTE. (R
2 = 0.669,
p < 0.001).
Another possible explanation for the correlation between decreased E/E′ and worsening of EIs is that the decreased LV filling pressure in patients with compromised LV cavity, as in hypertrophic cardiomyopathy, could worsen the severity in patients with existing D-LV. This finding could be applied to treatment plans from a clinical perspective, and additional confirmation is possible via repeated measurements in the same patients.
D-LV in echocardiography is a distinct finding of PH which needs to be understood for its hemodynamic interpretation. In our study, the most important factor for D-LV was E/E′, which indicates that the LV filling pressure is more important than the previously known factors, such as the severity of PH, i.e., RVSP or RVSP/systolic BP or TAPSE.
There were several limitations to our study. First, we did not perform cardiac catheterization to measure RVSP directly, which could decrease the accuracy of the study. Second, this study was conducted in a single center which could cause sampling bias. Third, there are not enough previous studies on EIs to limit the selection of confounding variables.
In conclusion, increased PAP, decreased TAPSE, E/E′, and age were found to be associated with the presence of D-LV in patients with increased PAP that were assessed by echocardiography. Moreover, for patients with D-LV, E/E′ was the only factor that was related to the severity of D-LV, as defined by the EIs.








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download