Abstract
Background
Calcitonin measurement is pivotal in the management of medullary thyroid carcinoma (MTC), but several pitfalls can affect its reliability. Other potential markers have been proposed, and procalcitonin (ProCT) has been reported as promising. The present study was undertaken to summarize the published data and provide more robust estimates on the reliability of ProCT as marker in the management of patients with MTC.
Methods
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The sources comprised studies published through May 2018. Original articles that reported series of MTC patients undergone ProCT during postoperative follow-up were searched. A random-effects model was used for statistical pooling of the data. The I2 index was used to quantify the consistency among the studies. The Egger test evaluated the possible presence of significant publication bias. Quality assessment of the studies was performed according to Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2).
Results
According to inclusion and exclusion criteria five papers, reporting 296 MTC patients undergone ProCT evaluation, were finally selected. The number of MTC with recurrence was 140. The pooled sensitivity of ProCT in detecting recurrence was 96% (95% confidence interval [CI], 92% to 99%), with neither heterogeneity (I2=0%) nor publication bias (Egger test, 3.16; P=0.99). The pooled specificity was 96% (95% CI, 87% to 100%) with mild heterogeneity (I2=66.6%), while Egger test was not calculable.
Medullary thyroid carcinoma (MTC) accounts for up to 5% of thyroid cancers, four in five cases occur as a sporadic and the remaining 20% are part of familial disorders [1]. The detection of MTC still represents a challenge and not infrequently MTC is only diagnosed histologically after surgery done for nodular goiter. This is due to several reasons [234]. In particular, several technical pitfalls can affect the measurement of serum calcitonin (CT) which represents the main presurgical and postoperative serum marker of MTC [4]; no fixed CT thresholds to diagnose and exclude MTC are available and non-specific increase of CT in several non-thyroidal conditions may exist [1]; MTC with undetectable CT is rarely reported [5]. Thus, further markers are advocated to better manage MTC cases before and after their initial treatment [1].
Recently, the measurement of procalcitonin (ProCT), a 116-aminoacids peptide product by thyroid C-cells as the precursor of CT, has been used in the management of MTC with good preliminary results [6]. ProCT is a very stable protein featuring a concentration-independent in vivo half-life of 20 to 24 hours [5]. The intellectual property for commercial PCT assays is licensed out by a single company, and therefore, commercially available assays are quite identical and well comparable. Also, sample for ProCT does not need to be kept cool on ice during the entire process chain. These characteristics give ProCT great potential to become a marker to manage MTC patients for clinical practice. However, while a systematic review was published in the past [6], no meta-analyses have been reported to better assess the reliability of ProCT in the identification of recurrent cancers and disease-free cases. This might represent a limitation for a larger diffusion of ProCT use.
Aim of the present systematic review and meta-analysis was to obtain more robust evidence in the performance of ProCT to detect recurrent MTC (hereafter REC) and identify MTC patients with no evidence of disease (NED). Based on the available data we also performed a meta-analysis of sensitivity and specificity of ProCT in this setting.
The present systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [7]; the PRISMA checklist can be found as Supplemental Table S1.
This article does not contain examinations performed on human participants. Then, ethical approval was not necessary.
A comprehensive literature search of the PubMed/MEDLINE and Scopus databases was conducted. Initially, we searched the largest possible number of papers reporting the use of ProCT in MTC. Then, we searched for studies evaluating the accuracy of ProCT during the postoperative follow-up of MTC. The initial search algorithm comprised the following terms: thyroid, procalcitonin, thyroid nodule (MeSH), and “Thyroid cancer, medullary” (supplementary concept). A beginning date limit was not used, the search extended through May, 2018, and no language restriction was used. To identify additional studies and expand our search, the references of the retrieved articles were screened.
Original articles that investigated ProCT during follow-up of patients treated for MTC were eligible for inclusion. The main exclusion criteria were articles that did not provide clear study characteristics and reports with overlapping patient data. The two authors independently reviewed the titles and abstracts of the retrieved articles, applying the inclusion and exclusion criteria. Then, the two researchers independently reviewed the full text of the remaining articles to determine whether they were suitable for inclusion. Discordances were resolved by consensus between the authors.
For each study that was included, information was extracted concerning the study itself (such as authors, year of publication, journal, and country of origin), the overall number of MTC undergone ProCT evaluation during follow-up, the number of REC and NED, and the rate true/false positive and true/false negative cases at ProCT test.
For statistical pooling of the data, the DerSimonian and Laird [8] method (a random-effects model) was used. In this model, pooled data represent weighted averages related to the sample size of the individual studies. The fixed-effects model was used. Pooled data were presented with 95% confidence intervals (CIs) and displayed using a forest plot. I2 index was used to quantify the heterogeneity among the studies, and a significant heterogeneity was defined as an I2 value >50% [9]. Egger test was carried out to evaluate the possible presence of a significant publication bias [10]. Statistical significance was set at P<0.01. All analyses were performed using the StatsDirect statistical software version (StatsDirect Ltd., Altrincham, UK). Quality assessment was performed by the two authors according to Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) [11].
The comprehensive computer-based literature search yielded 102 articles, of which some duplicates. From which a review of titles, abstracts, and full texts, 84 articles were excluded. Eight articles were initially eligible for the meta-analysis, and their full text was evaluated [1213141516171819]. Two articles were excluded due to not available data [12], and because they used a not commercialized ProCT test, different from that of the other articles [13]. Among the remaining six studies, one was excluded for overlapping data [16], five were finally included in the meta-analysis [1415171819]. Fig. 1 details the flowchart of article selection.
The most relevant paper was published by Algeciras-Schimnich et al. [14] who collected a series of 131 MTC patients of whom 43 REC and 42 NED, and ProCT >0.15 ng/mL in 42/43 REC and <0.15 ng/mL in 42/42 NED. Among these patients, those with stable disease during follow-up had the lowest ProCT levels. Kratzsch et al. [18] compared ProCT to several CT assays in different groups of patients with hypercalcitoninemia, including also recurrent/persistent MTC, and ProCT had the highest diagnostic sensitivity for REC MTC. One paper from Poland recorded 87% specificity and 100% sensitivity [15]. A study by Walter et al. [17] evaluated 69 REC MTC patients and found that ProCT was detectable in 67/69. In the most recently published paper [19], ProCT showed good performance detecting 11/12 REC and identifying 42/43 NED. Table 1 illustrates the results of the included papers. Quality assessment is reported in Table 2.
At final follow-up, the total number of MTC with proven recurrence was 140. The pooled sensitivity of ProCT in detecting REC was 96% (95% CI, 92% to 99%), ranging from 92% to 100% (Fig. 2). This finding showed complete absence of heterogeneity (I2=0%; 95% CI, 0% to 64.1%). Also, no publication bias was found (Egger test, 3.16; 95% CI, −1.13 to 1.13; P=0.99).
The number of MTC patients classified as NED in the articles was 108 in three papers because two papers did not report NED MTC cases [1718]. The pooled specificity was 96% (95% CI, 87% to 100%) ranging from 87% to 100% (Fig. 3). This result was affected by mild heterogeneity (I2=66.6%; 95% CI, 0% to 88.3%), and Egger test was not calculable.
Traditionally, MTC patients are treated by thyroidectomy and central (with or without lateral) neck-dissection, and after surgery CT is the pivotal tumor marker. However, several limits affect CT accuracy. High CT might be observed in C-cell hyperplasia, non-thyroidal neoplasia (neuroendocrine tumors, leukemias, systemic mastocytosis, small cell lung, breast and pancreatic cancer) renal failure, endocrine disfunctions (hyperparathyroidism, autoimmune thyroiditis), and physiologic conditions (pregnancy, lactation, neonatal period) [202122232425]. Also, CT is determined by several analytical tests with different immunoreactive isoforms and fragments which leads to poor comparability of results [26272829]. In addition, even if CT stimulation may improve its reliability, there is high variability of results from different articles in different populations. For all these reasons, the management of MTC in clinical practice may hide some pitfalls. In example, some MTC patients show a persistent biochemical disease (i.e., detectable CT and absence of proven structural disease). In these cases, international guidelines, due to the lack of evidence on CT determination and because of the precision of imaging tools, recommend to not use imaging in presence of CT below 150 pg/mL and do not specify reference ranges of stimulated serum CT to be adopted [1]. Thus, other MTC serum markers have been proposed, and ProCT has appeared as promising [6]. Interesting papers evaluated the role of ProCT in terms of prognosis and diagnosis of MTC with encouraging findings [3031]. However, further studies are needed to reach evidence on its potential use for the initial assessment of MTC.
In the present systematic review, we found papers describing MTC patients undergone ProCT during their postoperative follow-up and clinically assessed as REC or NED after adequate follow-up over time. Of high relevance, very high sensitivity of ProCT was found to detect recurrent/persistent disease and this finding represents a significant proof that ProCT can be used to detect persistent/recurrent disease. Also, specificity of ProCT in identifying NED MTC was high; however, due to the lack of data in some papers, this was calculated on a pooled series from only three papers. Both findings should indicate that ProCT performance in managing MTC patients after their initial treatment is excellent. Some of these studies suggested that ProCT should be used in combination to CT, especially in patients with mild to moderate increase of CT levels or those with inconsistent or fluctuating CT levels [141819]. In particular, in the study by Algeciras-Schimnich et al. [14], there were 91 MTC (eight newly diagnosed MTC, 40 MTC with detectable stable postoperative CT, and 43 MTC with structural recurrence), both CT and ProCT detected 83 of these 91, there were seven cases missed by CT and other seven missed by ProCT but one case was missed by both tests. All in all, the combined use of CT and ProCT reached a sensitivity of 98.9% (90/91 cases). Following the present results, MTC patients should be followed up over time by the combined evaluation of ProCT and CT.
As a limitation for the use of ProCT in MTC patients, we have to consider that severe inflammations, such as sepsis, may cause elevations of ProCT by tissues that do not normally transcribe the CT gene [32]. One study showed that ProCT can be used for monitoring sepsis in a patient affected by MTC [33]. On the other hand, whether common bacterial infections pose difficulties in the interpretation of ProCT levels from MTC patients is still to be proven.
Limitations and strengths of the present review have to be discussed. First, the final number of MTC cases included in the meta-analysis is suboptimal and a larger number would be desirable. Second, while the pooled sensitivity was statistically very strong (and was calculated in true REC patients), the pooled specificity was good but was calculated in only three articles. Third, the included papers reported different ProCT thresholds (receiver operating characteristic [ROC] derived or not), and this could introduce a significant bias for both sensitivity and specificity; unfortunately, original data were not available for each paper precluding a cumulative ROC curve analysis. Forth, all papers used the same assay, and this represents a significant strength. Fifth, the selected papers mainly refer to sporadic MTC and cannot be directly translated to patients carrying hereditary MTC.
In conclusion, the present meta-analysis was obtained selecting papers designed to assess REC and NED MTC patients. Importantly, these studies adopted ProCT assays employing the same commercially available assay and were conducted according to a state-of-the-art work-up protocol. The herein recorded pooled sensitivity and specificity of ProCT in detecting REC and NED cases were very high and this forms an evidence that ProCT can be used to manage these patients. Further studies using ProCT in combination to CT are needed.
References
1. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015; 25:567–610. PMID: 25810047.

2. Trimboli P, Treglia G, Guidobaldi L, Romanelli F, Nigri G, Valabrega S, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta-analysis. Clin Endocrinol (Oxf). 2015; 82:280–285. PMID: 25047365.

3. Pusztaszeri MP, Bongiovanni M, Faquin WC. Update on the cytologic and molecular features of medullary thyroid carcinoma. Adv Anat Pathol. 2014; 21:26–35. PMID: 24316908.

4. Trimboli P, Giovanella L, Crescenzi A, Romanelli F, Valabrega S, Spriano G, et al. Medullary thyroid cancer diagnosis: an appraisal. Head Neck. 2014; 36:1216–1223. PMID: 23955938.

5. Trimboli P, Giovanella L. Serum calcitonin negative medullary thyroid carcinoma: a systematic review of the literature. Clin Chem Lab Med. 2015; 53:1507–1514. PMID: 25781697.

6. Trimboli P, Seregni E, Treglia G, Alevizaki M, Giovanella L. Procalcitonin for detecting medullary thyroid carcinoma: a systematic review. Endocr Relat Cancer. 2015; 22:R157–R164. PMID: 25934688.

7. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009; 6:e1000100. PMID: 19621070.

8. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188. PMID: 3802833.

9. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.

10. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56:455–463. PMID: 10877304.

11. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536. PMID: 22007046.

12. Bihan H, Becker KL, Snider RH, Nylen E, Vittaz L, Lauret C, et al. Calcitonin precursor levels in human medullary thyroid carcinoma. Thyroid. 2003; 13:819–822. PMID: 14558925.

13. Bolko P, Manuszewska-Jopek E, Michalek K, Wasko R, Jaskula M, Sowinski J. Efficacy of procalcitonin measurement in patients after total thyroidectomy due to medullary thyroid carcinoma. Arch Immunol Ther Exp (Warsz). 2003; 51:415–419. PMID: 14692663.
14. Algeciras-Schimnich A, Preissner CM, Theobald JP, Finseth MS, Grebe SK. Procalcitonin: a marker for the diagnosis and follow-up of patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2009; 94:861–868. PMID: 19088163.

15. Kaczka K, Mikosinski S, Fendler W, Jalocha-Kaczka A, Pomorski L. Can procalcitonin be useful for medullary thyroid cancer? Endokrynol Pol. 2010; 61:430–436. PMID: 21049453.
16. Kaczka K, Mikosinski S, Fendler W, Celnik A, Pomorski L. Calcitonin and procalcitonin in patients with medullary thyroid cancer or bacterial infection. Adv Clin Exp Med. 2012; 21:169–178. PMID: 23214280.
17. Walter MA, Meier C, Radimerski T, Iten F, Kranzlin M, Muller-Brand J, et al. Procalcitonin levels predict clinical course and progression-free survival in patients with medullary thyroid cancer. Cancer. 2010; 116:31–40. PMID: 19890958.

18. Kratzsch J, Petzold A, Raue F, Reinhardt W, Brocker-Preuss M, Gorges R, et al. Basal and stimulated calcitonin and procalcitonin by various assays in patients with and without medullary thyroid cancer. Clin Chem. 2011; 57:467–474. PMID: 21159900.

19. Trimboli P, Lauretta R, Barnabei A, Valabrega S, Romanelli F, Giovanella L, et al. Procalcitonin as a postoperative marker in the follow-up of patients affected by medullary thyroid carcinoma. Int J Biol Markers. 2018; 33:156–160. PMID: 29707993.

20. Giovanella L, Imperiali M, Ferrari A, Palumbo A, Lippa L, Peretti A, et al. Thyroid volume influences serum calcitonin levels in a thyroid-healthy population: results of a 3-assay, 519 subjects study. Clin Chem Lab Med. 2012; 50:895–900. PMID: 22628334.

21. Leboulleux S, Baudin E, Travagli JP, Schlumberger M. Medullary thyroid carcinoma. Clin Endocrinol (Oxf). 2004; 61:299–310. PMID: 15355445.

22. Niccoli P, Conte-Devolx B, Lejeune PJ, Carayon P, Henry JF, Roux F, et al. Hypercalcitoninemia in conditions other than medullary cancers of the thyroid. Ann Endocrinol (Paris). 1996; 57:15–21. PMID: 8734284.
23. Iacobone M, Niccoli-Sire P, Sebag F, De Micco C, Henry JF. Can sporadic medullary thyroid carcinoma be biochemically predicted? Prospective analysis of 66 operated patients with elevated serum calcitonin levels. World J Surg. 2002; 26:886–890. PMID: 12016469.

24. Silva OL, Broder LE, Doppman JL, Snider RH, Moore CF, Cohen MH, et al. Calcitonin as a marker for bronchogenic cancer: a prospective study. Cancer. 1979; 44:680–684. PMID: 476577.
25. Zaidi M, Moonga BS, Bevis PJ, Bascal ZA, Breimer LH. The calcitonin gene peptides: biology and clinical relevance. Crit Rev Clin Lab Sci. 1990; 28:109–174. PMID: 1963534.

26. Fugazzola L, Pinchera A, Luchetti F, Iacconi P, Miccoli P, Romei C, et al. Disappearance rate of serum calcitonin after total thyroidectomy for medullary thyroid carcinoma. Int J Biol Markers. 1994; 9:21–24. PMID: 8051432.

27. Becker KL, Snider RH, Silva OL, Moore CF. Calcitonin heterogeneity in lung cancer and medullary thyroid cancer. Acta Endocrinol (Copenh). 1978; 89:89–99. PMID: 211777.

28. Goltzman D, Tischler AS. Characterization of the immunochemical forms of calcitonin released by a medullary thyroid carcinoma in tissue culture. J Clin Invest. 1978; 61:449–458. PMID: 621283.

29. Austin LA, Heath H 3rd. Calcitonin: physiology and pathophysiology. N Engl J Med. 1981; 304:269–278. PMID: 7003392.
30. Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab. 2014; 99:2986–2994. PMID: 24840813.

31. Giovanella L, Imperiali M, Piccardo A, Taborelli M, Verburg FA, Daurizio F, et al. Procalcitonin measurement to screen medullary thyroid carcinoma: a prospective evaluation in a series of 2,705 patients with thyroid nodules. Eur J Clin Invest. 2018; 48:e12934. PMID: 29635700.

32. Becker KL, Nylen ES, White JC, Muller B, Snider RH Jr. Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis. A journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004; 89:1512–1525. PMID: 15070906.
33. Novotny AR, Luppa P, Rosenberg R, Schneider H, Maak M, Bartels H, et al. Procalcitonin can be used for monitoring sepsis in patients with medullary thyroid carcinoma. Thyroid. 2009; 19:1287–1289. PMID: 19785521.

Fig. 1
Flow-chart of search and selection of papers. ProCT, procalcitonin; MTC, medullary thyroid carcinoma.
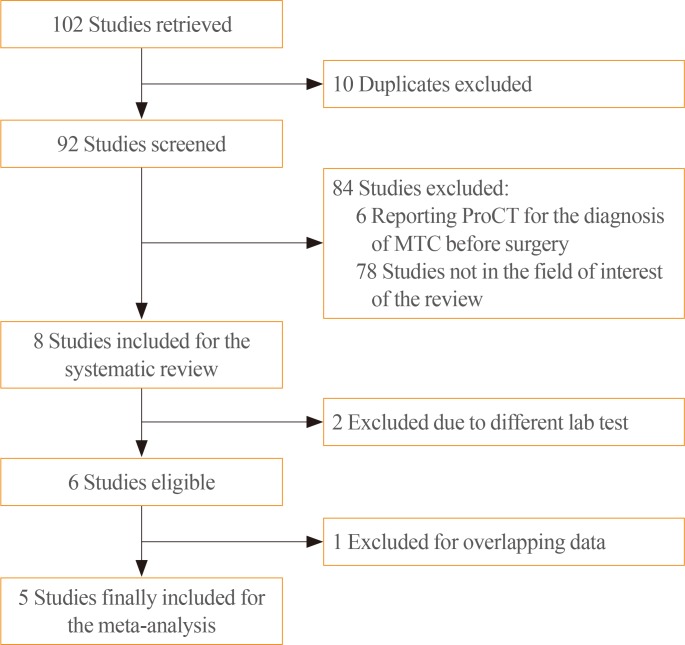
Fig. 2
Pooled sensitivity of procalcitonin in detecting REC MTC (random effect). REC, recurrent MTC; MTC, medullary thyroid carcinoma; CI, confidence interval.
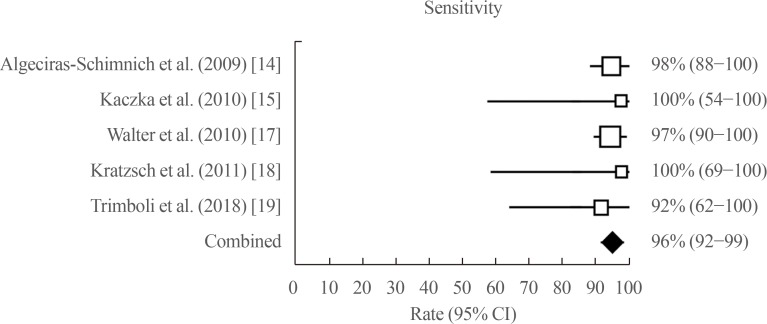
Fig. 3
Pooled specificity of procalcitonin in identifying NED MTC (random effect). NED, no evidence of disease; MTC, medullary thyroid carcinoma; CI, confidence interval.
Table 1
Original Articles Included in the Meta-Analysis
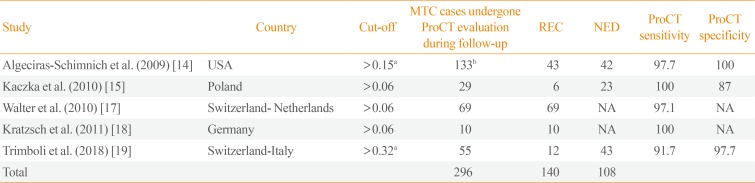
| Study | Country | Cut-off | MTC cases undergone ProCT evaluation during follow-up | REC | NED | ProCT sensitivity | ProCT specificity |
|---|---|---|---|---|---|---|---|
| Algeciras-Schimnich et al. (2009) [14] | USA | >0.15a | 133b | 43 | 42 | 97.7 | 100 |
| Kaczka et al. (2010) [15] | Poland | >0.06 | 29 | 6 | 23 | 100 | 87 |
| Walter et al. (2010) [17] | Switzerland-Netherlands | >0.06 | 69 | 69 | NA | 97.1 | NA |
| Kratzsch et al. (2011) [18] | Germany | >0.06 | 10 | 10 | NA | 100 | NA |
| Trimboli et al. (2018) [19] | Switzerland-Italy | >0.32a | 55 | 12 | 43 | 91.7 | 97.7 |
| Total | 296 | 140 | 108 |
MTC, medullary thyroid carcinoma; ProCT, procalcitonin; REC, recurrent MTC; NED, no evidence of disease; NA, not available.
aReceiver operating characteristic derived cut-off; bThis group included 8 newly diagnosed MTC, 40 MTC with postoperative detectable and stable calcitonin levels (assessed as stable disease), and 43 MTC with proved structural disease (REC disease).
Table 2
Quality Assessment of the Studies According to QUADAS-2
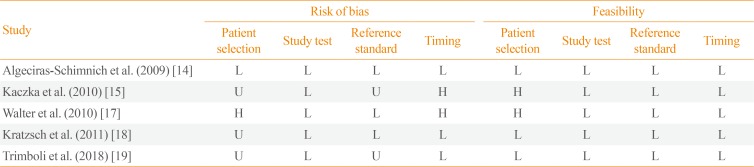
| Study | Risk of bias | Feasibility | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient selection | Study test | Reference standard | Timing | Patient selection | Study test | Reference standard | Timing | |
| Algeciras-Schimnich et al. (2009) [14] | L | L | L | L | L | L | L | L |
| Kaczka et al. (2010) [15] | L | U | U | H | H | L | L | L |
| Walter et al. (2010) [17] | L | L | H | H | H | L | L | L |
| Kratzsch et al. (2011) [18] | L | L | U | L | L | L | L | L |
| Trimboli et al. (2018) [19] | L | U | U | L | L | L | L | L |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download