Abstract
Autoimmune diabetes is a heterogeneous disease which can arise at any age. Subjects with adult-onset autoimmune diabetes who do not necessitate insulin-therapy for at least 6 months after diagnosis are demarcated as having latent autoimmune diabetes in adults (LADA). This condition is more heterogeneous than young-onset autoimmune diabetes and shares clinical and metabolic characteristics with both type 2 and type 1 diabetes. Patients with LADA are considered by having highly variable β-cell destruction, different degrees of insulin resistance and heterogeneous titre and pattern of islet autoantibody, suggesting different pathophysiological pathways partially explaining the heterogeneous phenotypes of LADA. To date the heterogeneity of LADA does not allow to establish a priori treatment algorithm and no specific guidelines for LADA therapy are available. These subjects are mostly treated as affected by type 2 diabetes, a factor that might lead to the progression to insulin-dependency quickly. A personalised medicine approach is necessary to attain optimal metabolic control and preserve β-cell function to decrease the risk of long-term diabetes complications. Recent data concerning the use of oral antidiabetic agents as dipeptidyl peptidase 4 inhibitors and glucagon-like peptide 1 receptor agonists indicate up-and-coming results in term of protect C-peptide levels and improving glycaemic control. This review summarises current knowledge on LADA, emphasising controversies regarding its pathophysiology and clinical features. Moreover, we discuss data available about novel therapeutic approaches that can be considered for prevention of β-cell loss in LADA.
Type 1 diabetes mellitus (T1DM) is an autoimmune disease derived from the selective destruction of insulin-secreting β-cells leading to requirement of insulin therapy [1]. This condition occurs in most cases during childhood or adolescence; however, some patients experience onset in adulthood [2]. A proportion of subjects with adult-onset autoimmune diabetes does not require insulin-therapy at the time of diagnosis and are clinically similar to patients with type 2 diabetes mellitus (T2DM). These patients, who were initially thought to be affected by T2DM, are defined as having latent autoimmune diabetes in adults (LADA) [345], a form of autoimmune diabetes, distinct from T1DM that shows an older age of onset and slower progression towards insulin requirement [678].
To diagnose LADA, the Immunology of Diabetes Society has established three main criteria including: (1) adult age of onset (>30 years); (2) presence of any islet cell autoantibody; and (3) absence of insulin requirement for at least 6 months after diagnosis [9]. However, the definition of LADA remains controversial and an open debate regarding these diagnostic criteria still exists. Thus, patients defined as having LADA are characterized by genetic, phenotypic, and immunological heterogeneity, highly variability of the β-cell destruction's rate and different degrees of insulin resistance and autoimmunity, likely due to differences in genetic and immune factors [101112]. Moreover, the great heterogeneity of LADA makes it difficult to determine an a priori algorithm for treatment. A personalised therapy for LADA should be implemented [13].
This review summarizes and discusses current knowledge about LADA, emphasizing the differences from both T1DM and T2DM. In addition, we examine the results of recent studies about novel therapeutic approaches that could prevent β-cell loss in LADA.
Most epidemiological studies suggest that adult-onset autoimmune diabetes is not rare as previously reported (Table 1) [51314151617]. Indeed, data collected from Italian registries show that the incidence of T1DM in subjects aged 30 to 49 years is similar to that of adolescents aged 15 to 19 years [18]. According to these data, studies among Caucasians from Northern Europe reported that approximately 40% of T1DM cases occur in people older than 30 years of age [2] and that the real incidence of this condition in subjects aged 15 to 34 years is up to three times higher than previously reported [19]. Data reported in LADA show that this is the most frequent form of adult-onset autoimmune diabetes and may account for 2% to 12% of all cases of diabetes in adult population [20]. Moreover, multicentre studies carried out in Europe, Asia, and North America, reported that 4% to 14% of patients diagnosed with T2DM are positive for T1DM associated autoantibodies which are diagnostic for LADA [45212223242526272829]. However, the prevalence of LADA seems to vary between different countries and populations, probably due to differences in study design and inclusion criteria, as well as different life-style and ethnicity. In Action LADA, a European multicentre study that evaluated over 6,000 adult-onset diabetes patients, the overall frequency of islet cell autoantibody positivity was reported in 9.7% of subjects with T2DM, even though consistent differences between patients living in North and South Europe were found, ranging between 4% and 10%. In accordance with these data, the NonInsulin Requiring Autoimmune Diabetes (NIRAD study) found a cumulative frequency of positivity for autoantibodies (glutamic acid decarboxylase [GAD] and/or protein tyrosine phosphatase [IA-2]) in 5% of Italian patients with T2DM [25]. Similar results emerge from studies carried out in Asia. In the multicentre China study a frequency of 5.9% was reported [30], whereas data in the Korean population show a prevalence ranging between 4.4% and 5.3% [15]. Regarding African-American, Hispanic, and Arab populations, recent studies have highlighted a lower prevalence of adult-onset autoimmune diabetes among these populations [531].
The spectrum of adult-onset diabetes involves all three forms of diabetes (T1DM, T2DM, and LADA) without defined parameters. The distribution of pathological factors, such as autoimmunity, insulin sensitivity and β-cell function, along a continuous line distinguishes categories of diabetes mellitus (Fig. 1) [132832]. When compared with classical T1DM, LADA appears like the other extreme of the autoimmune diabetes spectrum, whereby genetic susceptibility, autoimmune response and non-insulin-necessity presentation constitute a mild form of autoimmune diabetes with pathological features closer to those of T2DM than to those of adult T1DM, which is more similar to classical T1DM [13].
Data available on genetic susceptibility suggest that LADA shows a lower genetic component [101133] than T1DM. In particular, the human leukocyte antigen (HLA)-DRB1*04-DQB1*0302 and HLA-DRB1*0301-DQB1*0201, which are very common in young-onset T1DM and decrease in frequency with the increasing age at disease onset, are less frequent in LADA than in adult-onset T1DM [2534]. Similar findings have been observed for the Cyst1858Thr single-nucleotide polymorphism in the protein tyrosine phosphatase nonreceptor 22 (PTPN22) gene and the insulin variable number tandem repeat (INS VNTR) I/I genotype expression [3435]. Otherwise, the frequency of the cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) Ala49Gly polymorphism in exon 1 has been shown not to be associated with the age of onset of T1DM, suggesting a similar role in LADA susceptibility [36].
Another study carried out in Swedish and Finnish populations, showed that the frequency of T2DM associated CT/TT genotypes rs7903146 in the transcription factor 7 like 2 (TCF7L2) gene was increased in LADA subjects as in T2DM subjects [37], as well as genetic similarities with T1DM have been observed related to HLA, INS VNTR, and PTPN22 [38]. These results suggest that patients with LADA may share genetic features with both T1DM and T2DM which further supports the concept that LADA is an admixture of the two major types of diabetes [38].
However, it should be noted that most of these studies have investigated genes previously associated with young-onset T1DM or T2DM [1039404142]. Further studies are needed to better characterize the genetic susceptibility of LADA in order to establish preventive strategies as well as allow safe and effective therapies [43].
In T1DM activated T-cells to islet-cell proteins characterize the lymphomononuclear cell infiltration in the endocrine pancreas (insulitis), causing the progressive loss of insulin-secreting β-cells. On the other hand, islet cell autoantibodies seem to be an epiphenomenon rather than pathogenic factors in β-cells destruction, though their detection in plasma helps to diagnose autoimmune diabetes.
As a form of autoimmune diabetes, LADA is characterized by islet-cell specific autoantibody positivity and similar cell-mediated immune response although impairment of β-cells is slower than in classical T1DM [678]. Specifically, a recent study observed presence of insulitis by pancreatic scintigraphy using interleukin 2 (IL-2) radiolabelled with technetium-99m (99mTc) and contrast-enhanced magnetic resonance imaging, providing evidence for the presence of activated mononuclear cells in the pancreas of LADA subjects, particularly within 1 year of initiation of insulin therapy, similar to T1DM at diagnosis [44]. Moreover, increased pancreatic 99mTC-IL-2 uptake has been found in a subgroup of autoantibody-negative diabetic subjects, suggesting that insulitis may occur even in absence of islet-cell autoantibodies [45].
C-peptide, a marker of residual β-cell function, declines slower in LADA than in T1DM. Thus, a positive correlation between age at diagnosis of autoimmune diabetes and fasting C-peptide levels has been reported [46]. Indeed, LADA subjects show higher stimulated C-peptide values al all time-points following a mixed-meal tolerance than subjects with classical T1DM [12].
Islet-cell autoantibodies detectable in subjects with LADA are the same used to identify T1DM. Glutamic acid decarboxylase autoantibody (GADAs) is the most sensitive marker in both adult-onset T1DM and LADA, whereas insulin autoantibodies, protein tyrosine phosphatase IA-2 (IA-2A) and islet-specific zinc transporter isoform 8 (ZnT8) autoantibodies, which are detectable in younger T1DM patients, are positive only in a small percentage of LADA patients [47]. In the Action LADA study, 68.6% of subjects screened for diabetes-associated autoantibodies were positive for GADA only, 5% positive for IA-2A only and 2.3% positive for ZnT8A only, whereas at least two autoantibody types were found in 24.1% patients [4].
To sum up and as previously mentioned, LADA shows halfway pathological features between T1DM and T2DM. In a recent Italian work, it has been suggested that different pathophysiological could explain the heterogeneous phenotypes of LADA [13]. Based on this model, in patients with moderate genetic susceptibility to T1DM, specific immunological factors can trigger an autoimmune process against islet cell antigens marked by the appearance of GADAs leading to β-cell apoptosis and insulin deficiency. On the other hand, in obese subjects with genetic susceptibility to T2DM, the low-grade inflammation, typical of visceral adiposity, might trigger a low-grade autoimmune process marked by IA-2 autoantibodies positivity, causing loss of β-cell function and an impairment of insulin secretion (Fig. 2).
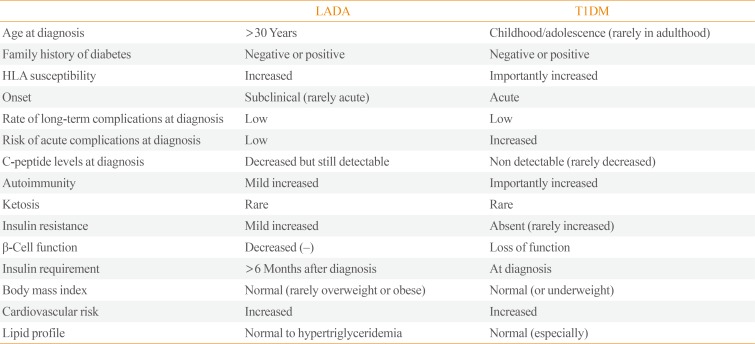
LADA shares clinical and metabolic features with T2DM and T1DM as part of a continuum of variable severity of immune and metabolic dysfunction [48], described as “end of the rainbow” (Tables 2, 3) [8].
Metabolic syndrome and its components (blood pressure, lipid profile, waist-hip ration) are less prevalent in LADA patients than in those with T2DM, both in Caucasians and non-Caucasians subjects [516]. Nevertheless, this assumption changes when LADA patients are compared with patients with T1DM. The BOTNIA study [22] demonstrated that components of metabolic syndrome have a higher prevalence in LADA than in classical T1DM. Similar results emerge from the Action LADA project [49]. Another study, carried out in Spain, showed that adiposity parameters, blood pressure and triglyceride levels are more elevated in LADA than in T1DM but lower than in T2DM [50]. Similarly, data collected among a cohort of subjects with LADA in the United Arab Emirates showed higher body mass index (BMI), waist circumference, systolic blood pressure and glycated hemoglobin (HbA1c) values [5]. On the other hand, Swedish and Norwegian registers report that overweight and obesity are associated with increased risk of LADA [37], particularly in combination with family history for diabetes. However, as previously discussed, LADA is characterized by a great phenotypic heterogeneity, witnessed by variable degrees of insulin resistance and insulin insufficiency.
Recent evidence shows that the titre of antibody positivity, especially GADA, can influence clinical characteristics of subjects with LADA [142528]. The NIRAD study [25] demonstrated the presence of a “bimodal distribution” of GADA titre in LADA sub-classifying two distinct forms of the disease. Patients with high GADA levels, typed as LADA 1, show more similar phenotypic features with T1DM (lower C-peptide levels, lower BMI, more ketosis prone) than with T2DM, whereas patients with low GADA titre, typed as LADA 2, are less ketosis and dyslipidemia prone as compared with LADA 1, but possess higher frequency of obesity, hypertension, dyslipidemia, and cardiovascular diseases. Similar results have been found in a Korean population where GADA levels showed a negative correlation with age of onset, total cholesterol, triglycerides, BMI, fasting and stimulated C-peptide, whereas a positive correlation was found with HbA1c and high density lipoprotein cholesterol [15]. The existence of two distinct subgroups of patients affected by LADA has been substantiated by genetic studies. PTPN22 risk genotype has been associated with a high GADA titre [51], whereas some but not all studies, have found an association between a lower GADA titre and the TCF7L2 risk allele for T2DM [52]. In addition, a higher frequency of other organ-specific autoantibodies (against thyroid peroxidase, steroid 21-hydroxylase, tissue, tissue transglutaminase, and parietal cell) was detected in patients with high GADA levels, highlighting a more extensive autoimmune process in this subpopulation than in those with a lower GADA titre [53]. Accordingly, several studies have testified an inverse relationship between GADA titre and C-peptide levels [54], as well as more pronounced traits of insulin deficiency were described in patients with higher GADA titre [25]. Another interesting finding concerns the relationship between clinical phenotypes of LADA and specific GADA binding patterns. Action LADA 12 [55] demonstrated an association between presence of N-terminally truncated GAD65 autoantibodies and the clinical phenotype of T1DM in patients with LADA. This may have important practical implications given the relatively large number of adult patients who are screened for GADA, both for diabetes classification and risk prediction for insulin therapy.
Even the number of islet autoantibodies in LADA appears to be associated with the intensity of autoimmune response. Indeed, the United Kingdom Prospective Diabetes Study (UKPDS) group and the NIRAD group, have noted that the number of islet cell autoantibodies was directly proportional to the intensity of autoimmune response, predicting more sever insulin insufficiency [214756].
Not only the titre, but also different patterns of antibody positivity could influence clinical features of LADA patients. In a cohort of approximately 17,000 subjects affected by LADA, the positivity for IA-2A only was associated with a clinical phenotype more similar to T2DM, whereas subjects positive for both IA-2A and GADA were more similar to T1DM [5]. Other authors observed that IA-2A was the only autoantibody that showed increased frequency with increasing BMI in patients affected by T2DM [57]. However, conflicting data have been reported. In the Action LADA 7 patients positive for at least two autoantibodies compared with those positive for a single antibody did not show relevant differences in demographic or clinical parameters [4]. Moreover, mixed results about the correlation between GADA titre and length of insulin-free period have been reported [14225458].
To date, only few data related to the development of late diabetic complications have been reported in patients with LADA.
There are only few studies related to the occurrence of microvascular complications (nephropathy, retinopathy, neuropathy) in LADA and controversial results have been reported, partly due to a substantial heterogeneity regarding disease duration of study's subjects. Limited to patients with a short disease duration, microvascular complications in LADA appear to be less frequent than in patients affected by T2DM. In particular, the Fremantle Diabetes Study [59], showed less frequency of microalbuminuria in patients with a recent diagnosis of LADA than in those with T2DM. In addition, in the same study an association between GADA positivity and a reduced risk of developing microalbuminuria during a follow-up period (<5 years) was found. In a study among Chinese subjects [60], it was observed that the onset of nephropathy and retinopathy is less common in patients with a recent diagnosis (<5 years) of LADA than in those affected by T2DM. However, these findings could be explained by the fact that subjects with T2DM are usually diagnosed later than subjects with LADA, being chronically expose to hyperglycaemia which may ultimately lead to microvascular complications. Thus, studies evaluating patients with a disease duration >5 years did not find differences between LADA and T2DM with respect to the prevalence of nephropathy or retinopathy, as well as no association was found between GADA positivity and rate of microvascular complications [616263]. However, the BOTNIA study [64] observed that patients with a disease duration >5 years had an increased risk of retinopathy; in addition, the rate of neuropathy appears more prevalent in LADA than in T2DM [59626465].
A lower risk of macrovascular complications—including coronary heart disease, stroke, peripheral artery disease—could be postulated on the basis of the healthier metabolic profile of patients with LADA respect to those with T2DM. However, current data showed similar cardiovascular outcomes in LADA and T2DM. In the BOTNIA study [64] no statistically significant differences were found in respect to coronary heart disease, stroke, overall mortality, and cardiovascular mortality between LADA and T2DM. Similar results emerged from other independent studies. The Fremantle Diabetes Study [59] did not evidenced different rate of cardiovascular disease and mortality in LADA versus T2DM, while the HUNT2 study observed similar increase of cardiovascular risk in patients positive for GAD antibodies and in those who were negative, compared to non-diabetic patients [66]. Altogether, these results suggest that different pathogenetic mechanisms might modulate the onset of macrovascular complications in LADA, regardless of the metabolic profile.
Impaired bone metabolism and increased risk of fractures have been observed in both T1DM and T2DM [67], partly due to reduction of bone formation. A recent cross-sectional study [68], showed that bone resorption is reduced in both LADA and T2DM compared to non-diabetic subjects whereas circulating sclerostin—which is an antagonist of the osteoblastic bone formation—is increased in T2DM only, probably due to the association between sclerostin levels and metabolic syndrome which is more prevalent in T2DM than in LADA. However, these data suggest that pathways involved in bone metabolism differ between these two types of diabetes and further studies are needed to clarify the pathogenesis of bone impairment and explore the potential role of sclerostin on bone fragility associated with diabetes.
To date, no specific guidelines for treatment of subjects affected by LADA have been published. Therefore, these subjects are mostly treated as affected by T2DM resulting in rapid progression to an insulin-dependent state [13], especially in patients who present with clinical and biochemical features closer to T1DM than T2DM [569]. In addition, a correct therapeutic strategy for LADA patients should aim to the preservation of residual β-cell function as well as improvement of glucometabolic control, in order to reduce the risk of long-term complications. Maintenance of β-cell function, as demonstrated by the Diabetes Control and Complication Trial, is indeed associated with a reduction of long teen diabetic complications [70].
A small randomized controlled trial, carried out in Japan, compared glibenclamide with insulin treatment in subjects affected by LADA. Results showed worsening metabolic control and progressive deterioration of β-cell function as measured by significant reduction of stimulated C-peptide ratio after 30 months follow-up in patients treated with sulphonylurea [71]. Similar results have been reported in the Tokyo study [72], a multicenter, randomized, nonblinded clinical study that evaluated 4,089 noninsulin-dependent LADA patients with a 5-year or shorter duration of diabetes. In this study Maruyama et al. [72] demonstrated that the progression rate to insulin-dependency in the sulphonylurea group was higher than that in the insulin group, and C-peptide during the oral glucose tolerance test was more preserved in patients undergoing insulin therapy. Altogether, these data suggest that sulphonylurea should not be used as first-line therapy in patients with LADA.
There insulin treatment is essential in all patients with complete β-cell loss and represents the most straightforward therapy for replacing missing endogenous insulin secretion [13]. However, subjects with a recent diagnosis of LADA are characterized by some degree of preservation of β-cell function as shown by higher C-peptide levels, and progress slower to absolute insulin dependency. Nevertheless, consistent data from randomized clinical trials highlight the importance of an early initiation of insulin therapy in LADA regardless of presence of some endogenous insulin secretion. The rationale behind this approach is to improve metabolic control while protecting β-cell function.
Even though the pathophysiological basis of the protective effect of insulin therapy is not well known, several preclinical studies showed that therapy with exogenous insulin would support β-cell function, reducing the hyperglycaemic stress [73] and decreasing severity of insulitis [74]. Other studies suggest the promotion of T helper 2 immunity and activation of insulin-specific regulatory T-cells mediated by the exposure to exogenous insulin [7576]. Moreover, a suppression of autoreactive T-cells through local release of regulatory cytokines has been suggested in patients treated with insulin [77].
An intervention intended to preserve β-cell function should be pursued in patients with LADA. Recent immune-intervention trials have achieved promising results in term of preserving stimulated C-peptide levels and improving glycaemic control [787980]. However, some drugs currently used for treatment of T2DM might be considered in LADA.
Dipeptidyl peptidase 4 (DPP-4) inhibitors represent a class of oral antidiabetic agents frequently used in T2DM which have been shown to preserve β-cell function and reduce insulitis in patients with T2DM as well as in mouse models of autoimmune diabetes [818283], suggesting that they might be a valuable treatment option in LADA. A recent randomized-controlled study conducted in China [84] has observed that treatment with sitagliptin in addition to insulin preserved C-peptide concentration better than insulin alone in patients with LADA over a 1-year period. Similarly, sitagliptin improved glycaemic control in adults with T1DM [85]. Furthermore, Johansen et al. [86] have reported that another DPP-4 inhibitor, linagliptin, attenuated decline of C-peptide in LADA patients over a 2-year study period. In a post hoc analysis of data pooled from five randomized, placebo-controlled studies [87], saxagliptin was effective in lowering blood glucose levels and well tolerated in GADA-positive patients. Moreover, compared with placebo saxagliptin appeared to increase C-peptide levels at 24 weeks follow-up.
Other interesting findings come from our study of a post hoc analysis investigating treatment with dulaglutide, a glucagon-like peptide 1 receptor agonist (GLP-1RA) [88] in patients with T2DM among whom there were some GAD antibody positive patients. The effectiveness of dulaglutide in subjects with LADA was indicated by reduction of HbA1c and increase of β-cell function, without affecting the rate of hypoglycaemia over 1-year observation period. This study is the first to indicate that dulaglutide is an effective anti-hyperglycaemic treatment in T2DM patients positive for GAD antibodies. Taking together these data highlight the potential long-term effects of DPP-4 and GLP-1RA in patients with LADA. However, further studies with larger cohort and longer treatment duration are needed to assess whether these therapies translate into a reduced progression to insulin dependence and diabetic complications [87].
Adult-onset autoimmune diabetes is a heterogeneous disease with clinical and metabolic features ranging from classical T1DM with onset from childhood to adult age, to LADA. When defined according to the criteria proposed by the Immunology of Diabetes Society, LADA also is characterized by significant degree of heterogeneity, encompassing different clinical phenotypes, ranging from prevalent insulin resistance to prevalent insulin deficiency associated with severe or mild markers of autoimmunity [47]. However, mid-way features between T1DM and T2DM are recognised in patients affected by LADA. The titre of antibody positivity [142528], especially GADA, as well as the recognition of different patterns of autoantibody positivity [5] can influence the clinical features of LADA and may be useful for diabetes classification and prediction of risk for insulin therapy.
Epidemiological studies show that LADA is a prevalent form of diabetes and may account for 2% to 12% of all cases of diabetes in adult population [20]. Moreover, 4% to 14% of patients diagnosed with T2DM are positive for T1DM associated autoantibodies which are diagnostic for LADA [4514212223242526272829]. For this reason, when diagnosing diabetes in adult age, a diagnosis of LADA should be always considered, especially when clinical features are suggestive of this form of diabetes.
To date, an a priori algorithm for treatment of LADA does not exist [13]. However, a personalized medicine approach is requested to achieve optimal metabolic control and preserve β-cell function, which is associated with a lower risk of occurrence of long-term diabetes complications.
In this regard, several data showed that insulin treatment, as well as DPP-4 agents, can sustain residual β-cell function [8182838485868788]. Insulin therapy (basal), at low dose can be prescribed to LADA patients with DPP-4 as an additional weapon, whereas sulphonylurea may hasten insulin dependency and should not be used as first-line therapy for patients with LADA (Fig. 3).
ACKNOWLEDGMENTS
The authors would like to thank Centro Internazionale Studi Diabete (CISD), a no-profit Association which has supported over the years our research in diabetes.
References
1. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010; 464:1293–1300. PMID: 20432533.
2. Molbak AG, Christau B, Marner B, Borch-Johnsen K, Nerup J. Incidence of insulin-dependent diabetes mellitus in age groups over 30 years in Denmark. Diabet Med. 1994; 11:650–655. PMID: 7955989.
3. Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes. 1993; 42:359–362. PMID: 8425674.

4. Hawa MI, Kolb H, Schloot N, Beyan H, Paschou SA, Buzzetti R, et al. Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: Action LADA 7. Diabetes Care. 2013; 36:908–913. PMID: 23248199.
5. Maddaloni E, Lessan N, Al Tikriti A, Buzzetti R, Pozzilli P, Barakat MT. Latent autoimmune diabetes in adults in the United Arab Emirates: clinical features and factors related to insulin-requirement. PLoS One. 2015; 10:e0131837. PMID: 26252955.

6. Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care. 2001; 24:1460–1467. PMID: 11473087.
7. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004; 27(Suppl 1):S5–S10. PMID: 14693921.
8. Leslie RD, Williams R, Pozzilli P. Clinical review: type 1 diabetes and latent autoimmune diabetes in adults. One end of the rainbow. J Clin Endocrinol Metab. 2006; 91:1654–1659. PMID: 16478821.
9. Fourlanos S, Dotta F, Greenbaum CJ, Palmer JP, Rolandsson O, Colman PG, et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia. 2005; 48:2206–2212. PMID: 16193284.

10. Sabbah E, Savola K, Ebeling T, Kulmala P, Vahasalo P, Ilonen J, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care. 2000; 23:1326–1332. PMID: 10977027.

11. Howson JM, Rosinger S, Smyth DJ, Boehm BO; ADBW-END Study Group, Todd JA. Genetic analysis of adult-onset autoimmune diabetes. Diabetes. 2011; 60:2645–2653. PMID: 21873553.

12. Hernandez M, Mollo A, Marsal JR, Esquerda A, Capel I, Puig-Domingo M, et al. Insulin secretion in patients with latent autoimmune diabetes (LADA): half way between type 1 and type 2 diabetes. Action LADA 9. BMC Endocr Disord. 2015; 15:1. PMID: 25572256.

13. Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol. 2017; 13:674–686. PMID: 28885622.

14. Maioli M, Pes GM, Delitala G, Puddu L, Falorni A, Tolu F, et al. Number of autoantibodies and HLA genotype, more than high titers of glutamic acid decarboxylase autoantibodies, predict insulin dependence in latent autoimmune diabetes of adults. Eur J Endocrinol. 2010; 163:541–549. PMID: 20603341.

15. Roh MO, Jung CH, Kim BY, Mok JO, Kim CH. The prevalence and characteristics of latent autoimmune diabetes in adults (LADA) and its relation with chronic complications in a clinical department of a university hospital in Korea. Acta Diabetol. 2013; 50:129–134. PMID: 20953640.

16. Lee SH, Kwon HS, Yoo SJ, Ahn YB, Yoon KH, Cha BY, et al. Identifying latent autoimmune diabetes in adults in Korea: the role of C-peptide and metabolic syndrome. Diabetes Res Clin Pract. 2009; 83:e62–e65. PMID: 19166794.

17. Park Y, Hong S, Park L, Woo J, Baik S, Nam M, et al. LADA prevalence estimation and insulin dependency during follow-up. Diabetes Metab Res Rev. 2011; 27:975–979. PMID: 22069296.

18. Bruno G, Runzo C, Cavallo-Perin P, Merletti F, Rivetti M, Pinach S, et al. Incidence of type 1 and type 2 diabetes in adults aged 30–49 years: the population-based registry in the province of Turin, Italy. Diabetes Care. 2005; 28:2613–2619. PMID: 16249528.
19. Rawshani A, Landin-Olsson M, Svensson AM, Nystrom L, Arnqvist HJ, Bolinder J, et al. The incidence of diabetes among 0-34 year olds in Sweden: new data and better methods. Diabetologia. 2014; 57:1375–1381. PMID: 24710965.
20. Naik RG, Palmer JP. Latent autoimmune diabetes in adults (LADA). Rev Endocr Metab Disord. 2003; 4:233–241. PMID: 14501174.
21. Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997; 350:1288–1293. PMID: 9357409.
22. Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, Nilsson A, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999; 48:150–157. PMID: 9892237.

23. Takeda H, Kawasaki E, Shimizu I, Konoue E, Fujiyama M, Murao S, et al. Clinical, autoimmune, and genetic characteristics of adult-onset diabetic patients with GAD autoantibodies in Japan (Ehime Study). Diabetes Care. 2002; 25:995–1001. PMID: 12032105.

24. Zinman B, Kahn SE, Haffner SM, O'Neill MC, Heise MA, Freed MI, et al. Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes. 2004; 53:3193–3200. PMID: 15561950.

25. Buzzetti R, Di Pietro S, Giaccari A, Petrone A, Locatelli M, Suraci C, et al. High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care. 2007; 30:932–938. PMID: 17392553.

26. Radtke MA, Midthjell K, Nilsen TI, Grill V. Heterogeneity of patients with latent autoimmune diabetes in adults: linkage to autoimmunity is apparent only in those with perceived need for insulin treatment. Results from the Nord-Trøndelag Health (HUNT) study. Diabetes Care. 2009; 32:245–250. PMID: 19001190.
27. Qi X, Sun J, Wang J, Wang PP, Xu Z, Murphy M, et al. Prevalence and correlates of latent autoimmune diabetes in adults in Tianjin, China: a population-based cross-sectional study. Diabetes Care. 2011; 34:66–70. PMID: 20876205.
28. Zhou Z, Xiang Y, Ji L, Jia W, Ning G, Huang G, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes. 2013; 62:543–550. PMID: 23086039.
29. Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet. 2014; 383:1084–1094. PMID: 24315621.

30. Yang Z, Wang K, Li T, Sun W, Li Y, Chang YF, et al. Childhood diabetes in China. Enormous variation by place and ethnic group. Diabetes Care. 1998; 21:525–529. PMID: 9571336.

31. Barinas-Mitchell E, Pietropaolo S, Zhang YJ, Henderson T, Trucco M, Kuller LH, et al. Islet cell autoimmunity in a triethnic adult population of the Third National Health and Nutrition Examination Survey. Diabetes. 2004; 53:1293–1302. PMID: 15111499.

32. Maddaloni E, Pozzilli P. Getting it right for people with LADA. Diabetes Voice. 2014; 59:31–32.
33. Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia. 2016; 59:13–20. PMID: 26498592.

34. Andersen MK, Lundgren V, Turunen JA, Forsblom C, Isomaa B, Groop PH, et al. Latent autoimmune diabetes in adults differs genetically from classical type 1 diabetes diagnosed after the age of 35 years. Diabetes Care. 2010; 33:2062–2064. PMID: 20805278.

35. Haller K, Kisand K, Pisarev H, Salur L, Laisk T, Nemvalts V, et al. Insulin gene VNTR, CTLA-4 +49A/G and HLA-DQB1 alleles distinguish latent autoimmune diabetes in adults from type 1 diabetes and from type 2 diabetes group. Tissue Antigens. 2007; 69:121–127. PMID: 17257313.

36. Van der Auwera BJ, Vandewalle CL, Schuit FC, Winnock F, De Leeuw IH, Van Imschoot S, et al. CTLA-4 gene polymorphism confers susceptibility to insulin-dependent diabetes mellitus (IDDM) independently from age and from other genetic or immune disease markers. The Belgian Diabetes Registry. Clin Exp Immunol. 1997; 110:98–103. PMID: 9353155.
37. Hjort R, Ahlqvist E, Carlsson PO, Grill V, Groop L, Martinell M, et al. Overweight, obesity and the risk of LADA: results from a Swedish case-control study and the Norwegian HUNT Study. Diabetologia. 2018; 61:1333–1343. PMID: 29589073.

38. Cervin C, Lyssenko V, Bakhtadze E, Lindholm E, Nilsson P, Tuomi T, et al. Genetic similarities between latent autoimmune diabetes in adults, type 1 diabetes, and type 2 diabetes. Diabetes. 2008; 57:1433–1437. PMID: 18310307.

39. Nistico L, Buzzetti R, Pritchard LE, Van der Auwera B, Giovannini C, Bosi E, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian Diabetes Registry. Hum Mol Genet. 1996; 5:1075–1080. PMID: 8817351.

40. Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004; 36:337–338. PMID: 15004560.

41. Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006; 38:320–323. PMID: 16415884.

42. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007; 316:889–894. PMID: 17434869.
43. Maddaloni E, Pozzilli P. SMART diabetes: the way to go (Safe and Multifactorial Approach to reduce the Risk for Therapy in diabetes). Endocrine. 2014; 46:3–5. PMID: 24381129.

44. Signore A, Capriotti G, Chianelli M, Bonanno E, Galli F, Catalano C, et al. Detection of insulitis by pancreatic scintigraphy with 99mTc-labeled IL-2 and MRI in patients with LADA (Action LADA 10). Diabetes Care. 2015; 38:652–658. PMID: 25665813.

45. Brooks-Worrell BM, Reichow JL, Goel A, Ismail H, Palmer JP. Identification of autoantibody-negative autoimmune type 2 diabetic patients. Diabetes Care. 2011; 34:168–173. PMID: 20855551.

46. Barker A, Lauria A, Schloot N, Hosszufalusi N, Ludvigsson J, Mathieu C, et al. Age-dependent decline of β-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes Obes Metab. 2014; 16:262–267. PMID: 24118704.

47. Lampasona V, Petrone A, Tiberti C, Capizzi M, Spoletini M, di Pietro S, et al. Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care. 2010; 33:104–108. PMID: 19808923.
48. Leslie RD, Kolb H, Schloot NC, Buzzetti R, Mauricio D, De Leiva A, et al. Diabetes classification: grey zones, sound and smoke: Action LADA 1. Diabetes Metab Res Rev. 2008; 24:511–519. PMID: 18615859.

49. Hawa MI, Thivolet C, Mauricio D, Alemanno I, Cipponeri E, Collier D, et al. Metabolic syndrome and autoimmune diabetes: Action LADA 3. Diabetes Care. 2009; 32:160–164. PMID: 18945926.

50. Mollo A, Hernandez M, Marsal JR, Esquerda A, Rius F, Blanco-Vaca F, et al. Latent autoimmune diabetes in adults is perched between type 1 and type 2: evidence from adults in one region of Spain. Diabetes Metab Res Rev. 2013; 29:446–451. PMID: 23483713.

51. Petrone A, Suraci C, Capizzi M, Giaccari A, Bosi E, Tiberti C, et al. The protein tyrosine phosphatase nonreceptor 22 (PTPN22) is associated with high GAD antibody titer in Latent autoimmune diabetes in adults: Non Insulin Requiring Autoimmune Diabetes (NIRAD) Study 3. Diabetes Care. 2008; 31:534–538. PMID: 18056891.
52. Zampetti S, Spoletini M, Petrone A, Capizzi M, Arpi ML, Tiberti C, et al. Association of TCF7L2 gene variants with low GAD autoantibody titre in LADA subjects (NIRAD Study 5). Diabet Med. 2010; 27:701–704. PMID: 20546291.

53. Zampetti S, Capizzi M, Spoletini M, Campagna G, Leto G, Cipolloni L, et al. GADA titer-related risk for organ-specific autoimmunity in LADA subjects subdivided according to gender (NIRAD study 6). J Clin Endocrinol Metab. 2012; 97:3759–3765. PMID: 22865904.

54. Genovese S, Bazzigaluppi E, Goncalves D, Ciucci A, Cavallo MG, Purrello F, et al. Clinical phenotype and beta-cell autoimmunity in Italian patients with adult-onset diabetes. Eur J Endocrinol. 2006; 154:441–447. PMID: 16498058.
55. Achenbach P, Hawa MI, Krause S, Lampasona V, Jerram ST, Williams AJK, et al. Autoantibodies to N-terminally truncated GAD improve clinical phenotyping of individuals with adult-onset diabetes: Action LADA 12. Diabetologia. 2018; 61:1644–1649. PMID: 29619531.

56. Bottazzo GF, Bosi E, Cull CA, Bonifacio E, Locatelli M, Zimmet P, et al. IA-2 antibody prevalence and risk assessment of early insulin requirement in subjects presenting with type 2 diabetes (UKPDS 71). Diabetologia. 2005; 48:703–708. PMID: 15765222.

57. Buzzetti R, Spoletini M, Zampetti S, Campagna G, Marandola L, Panimolle F, et al. Tyrosine phosphatase-related islet antigen 2(256-760) autoantibodies, the only marker of islet autoimmunity that increases by increasing the degree of BMI in obese subjects with type 2 diabetes. Diabetes Care. 2015; 38:513–520. PMID: 25567348.
58. Desai M, Cull CA, Horton VA, Christie MR, Bonifacio E, Lampasona V, et al. GAD autoantibodies and epitope reactivities persist after diagnosis in latent autoimmune diabetes in adults but do not predict disease progression: UKPDS 77. Diabetologia. 2007; 50:2052–2060. PMID: 17657474.

59. Myhill P, Davis WA, Bruce DG, Mackay IR, Zimmet P, Davis TM. Chronic complications and mortality in community-based patients with latent autoimmune diabetes in adults: the Fremantle Diabetes Study. Diabet Med. 2008; 25:1245–1250. PMID: 19046207.

60. Lu J, Hou X, Zhang L, Hu C, Zhou J, Pang C, et al. Associations between clinical characteristics and chronic complications in latent autoimmune diabetes in adults and type 2 diabetes. Diabetes Metab Res Rev. 2015; 31:411–420. PMID: 25448723.

61. Hawa MI, Buchan AP, Ola T, Wun CC, DeMicco DA, Bao W, et al. LADA and CARDS: a prospective study of clinical outcome in established adult-onset autoimmune diabetes. Diabetes Care. 2014; 37:1643–1649. PMID: 24722498.

62. Arikan E, Sabuncu T, Ozer EM, Hatemi H. The clinical characteristics of latent autoimmune diabetes in adults and its relation with chronic complications in metabolically poor controlled Turkish patients with type 2 diabetes mellitus. J Diabetes Complications. 2005; 19:254–258. PMID: 16112499.

63. Balme M, McAllister I, Davis WA, Davis TM. Retinopathy in latent autoimmune diabetes of adults: the Fremantle Diabetes Study. Diabet Med. 2002; 19:602–605. PMID: 12099965.

64. Isomaa B, Almgren P, Henricsson M, Taskinen MR, Tuomi T, Groop L, et al. Chronic complications in patients with slowly progressing autoimmune type 1 diabetes (LADA). Diabetes Care. 1999; 22:1347–1353. PMID: 10480781.

65. Wang C, Lu J, Lu W, Yu H, Jiang L, Li M, et al. Evaluating peripheral nerve function in asymptomatic patients with type 2 diabetes or latent autoimmune diabetes of adults (LADA): results from nerve conduction studies. J Diabetes Complications. 2015; 29:265–269. PMID: 25434703.

66. Olsson L, Grill V, Midthjell K, Ahlbom A, Andersson T, Carlsson S. Mortality in adult-onset autoimmune diabetes is associated with poor glycemic control: results from the HUNT Study. Diabetes Care. 2013; 36:3971–3978. PMID: 24130367.
67. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017; 13:208–219. PMID: 27658727.

68. Napoli N, Strollo R, Defeudis G, Leto G, Moretti C, Zampetti S, et al. Serum sclerostin and bone turnover in latent autoimmune diabetes in adults. J Clin Endocrinol Metab. 2018; 103:1921–1928. PMID: 29506222.

69. Zampetti S, Campagna G, Tiberti C, Songini M, Arpi ML, De Simone G, et al. High GADA titer increases the risk of insulin requirement in LADA patients: a 7-year follow-up (NIRAD study 7). Eur J Endocrinol. 2014; 171:697–704. PMID: 25213702.

70. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003; 26:832–836. PMID: 12610045.
71. Kobayashi T, Nakanishi K, Murase T, Kosaka K. Small doses of subcutaneous insulin as a strategy for preventing slowly progressive beta-cell failure in islet cell antibody-positive patients with clinical features of NIDDM. Diabetes. 1996; 45:622–626. PMID: 8621013.

72. Maruyama T, Tanaka S, Shimada A, Funae O, Kasuga A, Kanatsuka A, et al. Insulin intervention in slowly progressive insulin-dependent (type 1) diabetes mellitus. J Clin Endocrinol Metab. 2008; 93:2115–2121. PMID: 18397986.

73. Argoud GM, Schade DS, Eaton RP. Insulin suppresses its own secretion in vivo. Diabetes. 1987; 36:959–962. PMID: 3297890.

74. Jansen A, Rosmalen JG, Homo-Delarche F, Dardenne M, Drexhage HA. Effect of prophylactic insulin treatment on the number of ER-MP23+ macrophages in the pancreas of NOD mice. Is the prevention of diabetes based on beta-cell rest? J Autoimmun. 1996; 9:341–348. PMID: 8816969.
75. Fuchtenbusch M, Kredel K, Bonifacio E, Schnell O, Ziegler AG. Exposure to exogenous insulin promotes IgG1 and the T-helper 2-associated IgG4 responses to insulin but not to other islet autoantigens. Diabetes. 2000; 49:918–925. PMID: 10866043.
76. Tiittanen M, Huupponen JT, Knip M, Vaarala O. Insulin treatment in patients with type 1 diabetes induces upregulation of regulatory T-cell markers in peripheral blood mononuclear cells stimulated with insulin in vitro. Diabetes. 2006; 55:3446–3454. PMID: 17130491.

77. Schloot N, Eisenbarth GS. Isohormonal therapy of endocrine autoimmunity. Immunol Today. 1995; 16:289–294. PMID: 7662098.

78. Agardh CD, Cilio CM, Lethagen A, Lynch K, Leslie RD, Palmer M, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005; 19:238–246. PMID: 15993359.

79. Pozzilli P, Guglielmi C. Immunomodulation for the prevention of SPIDDM and LADA. Ann N Y Acad Sci. 2006; 1079:90–98. PMID: 17130536.

80. Agardh CD, Lynch KF, Palmer M, Link K, Lernmark A. GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia. 2009; 52:1363–1368. PMID: 19404608.
81. D'Alessio DA, Denney AM, Hermiller LM, Prigeon RL, Martin JM, Tharp WG, et al. Treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin improves fasting islet-cell function in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2009; 94:81–88. PMID: 18957505.
82. Foley JE, Bunck MC, Moller-Goede DL, Poelma M, Nijpels G, Eekhoff EM, et al. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naïve patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia. 2011; 54:1985–1991. PMID: 21547496.
83. Tian L, Gao J, Hao J, Zhang Y, Yi H, O'Brien TD, et al. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010; 151:3049–3060. PMID: 20444936.
84. Zhao Y, Yang L, Xiang Y, Liu L, Huang G, Long Z, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin maintains β-cell function in patients with recent-onset latent autoimmune diabetes in adults: one year prospective study. J Clin Endocrinol Metab. 2014; 99:E876–E880. PMID: 24432999.

85. Ellis SL, Moser EG, Snell-Bergeon JK, Rodionova AS, Hazenfield RM, Garg SK. Effect of sitagliptin on glucose control in adult patients with type 1 diabetes: a pilot, double-blind, randomized, crossover trial. Diabet Med. 2011; 28:1176–1181. PMID: 21923696.
86. Johansen OE, Boehm B, Grill V, Torjesen PA, Bhattacharya S, Patel S, et al. Beta-cell function in latent autoimmune diabetes in adults treated with linagliptin vs glimepiride: exploratory results from a 2-year double-blind randomized controlled study. Endocr Rev. 2012; 33(4 Suppl):SUN–LB1.
87. Buzzetti R, Pozzilli P, Frederich R, Iqbal N, Hirshberg B. Saxagliptin improves glycaemic control and C-peptide secretion in latent autoimmune diabetes in adults (LADA). Diabetes Metab Res Rev. 2016; 32:289–296. PMID: 26385269.

88. Pozzilli P, Leslie RD, Peters AL, Buzzetti R, Shankar SS, Milicevic Z, et al. Dulaglutide treatment results in effective glycaemic control in latent autoimmune diabetes in adults (LADA): a post-hoc analysis of the AWARD-2, -4 and -5 trials. Diabetes Obes Metab. 2018; 20:1490–1498. PMID: 29377522.

Fig. 1
Pathogenetic features and their relation to define diabetes. T1DM, type 1 diabetes mellitus; LADA, latent autoimmune diabetes in adults; T2DM, type 2 diabetes mellitus [32].
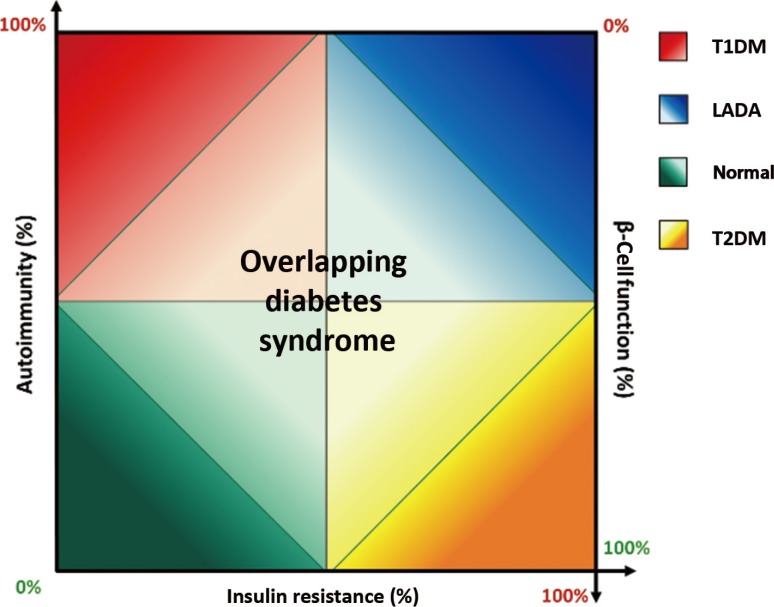
Fig. 2
Potential pathological pathways of latent autoimmune diabetes in adults (LADA). Modified from Buzzetti et al., with permission from Springer Nature [13]. TCF7L2, transcription factor 7 like 2; HLA, human leukocyte antigen; INS VNTR, insulin variable number tandem repeat; PTPN22, protein tyrosine phosphatase nonreceptor 22; GADA, glutamic acid decarboxylase autoantibody; IA-2A, protein tyrosine phosphatase IA-2.
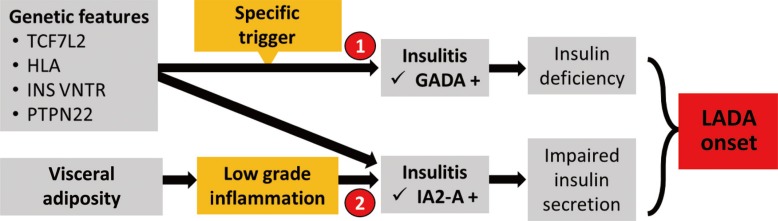
Fig. 3
Algorithm for diagnosis and therapy of latent autoimmune diabetes in adults (LADA). BMI, body mass index; T2DM, type 2 diabetes mellitus; GADA, glutamic acid decarboxylase autoantibody; DPP-4, dipeptidyl peptidase 4.
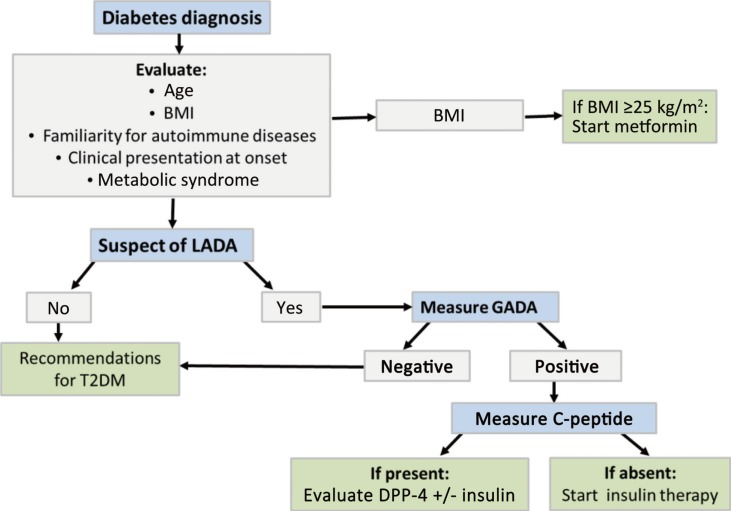
Table 1
Epidemiology of LADA [13]
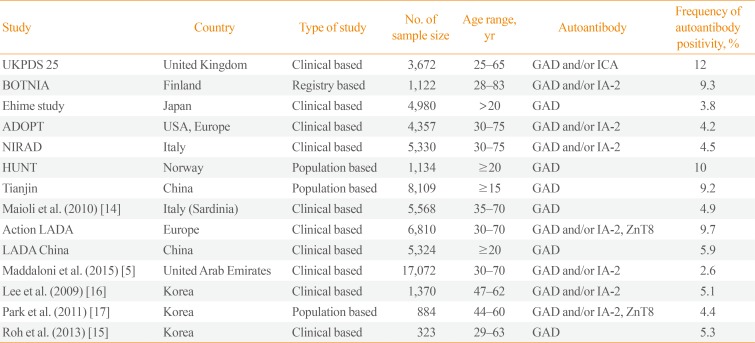
| Study | Country | Type of study | No. of sample size | Age range, yr | Autoantibody | Frequency of autoantibody positivity, % |
|---|---|---|---|---|---|---|
| UKPDS 25 | United Kingdom | Clinical based | 3,672 | 25–65 | GAD and/or ICA | 12 |
| BOTNIA | Finland | Registry based | 1,122 | 28–83 | GAD and/or IA-2 | 9.3 |
| Ehime study | Japan | Clinical based | 4,980 | >20 | GAD | 3.8 |
| ADOPT | USA, Europe | Clinical based | 4,357 | 30–75 | GAD and/or IA-2 | 4.2 |
| NIRAD | Italy | Clinical based | 5,330 | 30–75 | GAD and/or IA-2 | 4.5 |
| HUNT | Norway | Population based | 1,134 | ≥20 | GAD | 10 |
| Tianjin | China | Population based | 8,109 | ≥15 | GAD | 9.2 |
| Maioli et al. (2010) [14] | Italy (Sardinia) | Clinical based | 5,568 | 35–70 | GAD | 4.9 |
| Action LADA | Europe | Clinical based | 6,810 | 30–70 | GAD and/or IA-2, ZnT8 | 9.7 |
| LADA China | China | Clinical based | 5,324 | ≥20 | GAD | 5.9 |
| Maddaloni et al. (2015) [5] | United Arab Emirates | Clinical based | 17,072 | 30–70 | GAD and/or IA-2 | 2.6 |
| Lee et al. (2009) [16] | Korea | Clinical based | 1,370 | 47–62 | GAD and/or IA-2 | 5.1 |
| Park et al. (2011) [17] | Korea | Population based | 884 | 44–60 | GAD and/or IA-2, ZnT8 | 4.4 |
| Roh et al. (2013) [15] | Korea | Clinical based | 323 | 29–63 | GAD | 5.3 |
Adapted from Buzzetti et al., with permission from Springer Nature [13].
LADA, latent autoimmune diabetes in adults; UKPDS, United Kingdom Prospective Diabetes Study; GAD, glutamic acid decarboxylase; ICA, islet cell; IA-2, protein tyrosine phosphatase; NIRAD, NonInsulin Requiring Autoimmune Diabetes; ZnT8, islet-specific zinc transporter isoform 8.
Table 2
Differences in Clinical and Genetic Features between LADA and T2DM
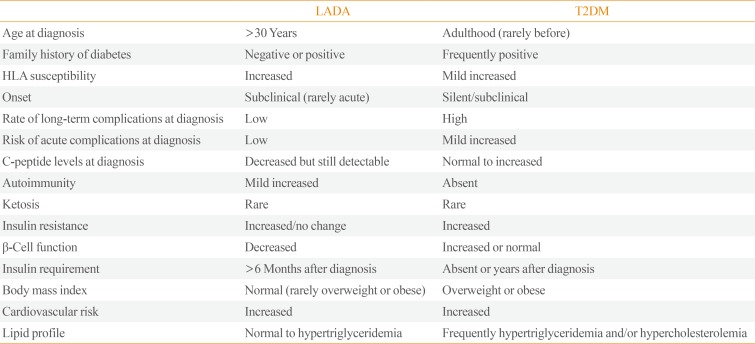
Table 3
Differences in Clinical and Genetic Features between LADA and T1DM




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download