1. National Cancer Control Programme, Ministry of Health, Sri Lanka. Cancer Incidence Data: Sri Lanka 2010. Colombo: National Cancer Control Programme, Ministry of Health, Sri Lanka;2016.
2. Sirisena ND, Dissanayake VHW. Cancer genetics and the surgeon: new frontiers. Sri Lanka J Surg. 2014; 32:12–19.
3. Qin TT, Chen T, Zhang Q, Du HN, Shu YQ, Luo K, et al. Association between BRCA1 rs799917 polymorphism and breast cancer risk: a meta-analysis of 19,878 subjects. Biomed Pharmacother. 2014; 68:905–910. PMID:
25194442.

4. Yu KD, Di GH, Fan L, Hu Z, Chen AX, Shao ZM. Caution regarding genotyping methodology for a tri-allelic polymorphism in the novel breast cancer susceptibility gene NQO2. Breast Cancer Res Treat. 2009; 118:647–649. PMID:
19495956.

5. Sirisena ND, Adeyemo A, Kuruppu AI, Neththikumara N, Samaranayake N, Dissanayake VHW. Genetic determinants of sporadic breast cancer in Sri Lankan women. BMC Cancer. 2018; 18:180. PMID:
29433565.

6. Zhang J, Zhang M, Jiang W, Wang L, Fu Z, Li D, et al. B7-H4 gene polymorphisms are associated with sporadic breast cancer in a Chinese Han population. BMC Cancer. 2009; 9:394. PMID:
19903360.

7. Oliveira C, Lourenço GJ, Silva PM, Cardoso-Filho C, Favarelli MH, Gonçales NS, et al. Polymorphisms in the 5′- and 3′-untranslated region of the VEGF gene and sporadic breast cancer risk and clinicopathologic characteristics. Tumour Biol. 2011; 32:295–300. PMID:
20981515.

8. Nickels S, Truong T, Hein R, Stevens K, Buck K, Behrens S, et al. Evidence of gene-environment interactions between common breast cancer susceptibility loci and established environmental risk factors. PLoS Genet. 2013; 9:e1003284. PMID:
23544014.
9. Lei J, Rudolph A, Moysich KB, Behrens S, Goode EL, Bolla MK, et al. Genetic variation in the immunosuppression pathway genes and breast cancer susceptibility: a pooled analysis of 42,510 cases and 40,577 controls from the Breast Cancer Association Consortium. Hum Genet. 2016; 135:137–154. PMID:
26621531.
10. Lum SS, Chua HW, Li H, Li WF, Rao N, Wei J, et al. MDM2 SNP309 G allele increases risk but the T allele is associated with earlier onset age of sporadic breast cancers in the Chinese population. Carcinogenesis. 2008; 29:754–761. PMID:
18281248.

11. Jia YM, Xie YT, Wang YJ, Han JY, Tian XX, Fang WG. Association of genetic polymorphisms in CDH1 and CTNNB1 with breast cancer susceptibility and patients' prognosis among Chinese Han women. PLoS One. 2015; 10:e0135865. PMID:
26285011.

12. Xu F, Li D, Zhang Q, Fu Z, Zhang J, Yuan W, et al. ICOS gene polymorphisms are associated with sporadic breast cancer: a case-control study. BMC Cancer. 2011; 11:392. PMID:
21917182.

13. Cao YW, Fu XG, Wan GX, Yu SY, Cui XB, Li L, et al. BRCA1 gene exon 11 mutations in Uighur and Han women with early-onset sporadic breast cancer in the northwest region of China. Asian Pac J Cancer Prev. 2014; 15:4513–4518. PMID:
24969878.

14. Sirisena ND, Dissanayake VHW. Focusing attention on ancestral diversity within genomics research: a potential means for promoting equity in the provision of genomics based healthcare services in developing countries. J Community Genet. 2017; 8:275–281. PMID:
28699077.

15. Chen S, Zhang Q, Shen L, Liu Y, Xu F, Li D, et al. Investigation of CD28 gene polymorphisms in patients with sporadic breast cancer in a Chinese Han population in Northeast China. PLoS One. 2012; 7:e48031. PMID:
23133541.

16. Lokuhetty MD, Ranaweera GG, Wijeratne MD, Wickramasinghe KH, Sheriffdeen AH. Profile of breast cancer in a group of women in a developing country in South Asia: is there a difference? World J Surg. 2009; 33:455–459. PMID:
19123026.

17. Ratnatunga N, Liyanapathirana LV. Hormone receptor expression and Her/2neu amplification in breast carcinoma in a cohort of Sri Lankans. Ceylon Med J. 2007; 52:133–136. PMID:
18286776.

18. Dayem Ullah AZ, Lemoine NR, Chelala C. A practical guide for the functional annotation of genetic variations using SNPnexus. Brief Bioinform. 2013; 14:437–447. PMID:
23395730.

19. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81:559–575. PMID:
17701901.

20. Wang K, Xu L, Pan L, Xu K, Li G. The functional BRCA1 rs799917 genetic polymorphism is associated with gastric cancer risk in a Chinese Han population. Tumour Biol. 2015; 36:393–397. PMID:
25266802.

21. Hasan TN, Shafi G, Syed NA, Alsaif MA, Alsaif AA, Alshatwi AA. Lack of association of BRCA1 and BRCA2 variants with breast cancer in an ethnic population of Saudi Arabia, an emerging high-risk area. Asian Pac J Cancer Prev. 2013; 14:5671–5674. PMID:
24289560.

22. Huo X, Lu C, Huang X, Hu Z, Jin G, Ma H, et al. Polymorphisms in BRCA1, BRCA1-interacting genes and susceptibility of breast cancer in Chinese women. J Cancer Res Clin Oncol. 2009; 135:1569–1575. PMID:
19484476.

23. Nicoloso MS, Sun H, Spizzo R, Kim H, Wickramasinghe P, Shimizu M, et al. Single-nucleotide polymorphisms inside microRNA target sites influence tumor susceptibility. Cancer Res. 2010; 70:2789–2798. PMID:
20332227.

24. Choi JY, Barlow WE, Albain KS, Hong CC, Blanco JG, Livingston RB, et al. Nitric oxide synthase variants and disease-free survival among treated and untreated breast cancer patients in a Southwest Oncology Group clinical trial. Clin Cancer Res. 2009; 15:5258–5266. PMID:
19671875.

25. Yu KD, Huang AJ, Fan L, Li WF, Shao ZM. Genetic variants in oxidative stress-related genes predict chemoresistance in primary breast cancer: a prospective observational study and validation. Cancer Res. 2012; 72:408–419. PMID:
22147260.

26. Li D, Fu Z, Chen S, Yuan W, Liu Y, Li L, et al. HVEM gene polymorphisms are associated with sporadic breast cancer in Chinese women. PLoS One. 2013; 8:e71040. PMID:
23976978.

27. Deng L, Chen J, Zhong XR, Luo T, Wang YP, Huang HF, et al. Correlation between activation of PI3K/AKT/mTOR pathway and prognosis of breast cancer in Chinese women. PLoS One. 2015; 10:e0120511. PMID:
25816324.

28. Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008; 68:9221–9230. PMID:
19010894.

29. Erturk E, Cecener G, Polatkan V, Gokgoz S, Egeli U, Tunca B, et al. Evaluation of genetic variations in miRNA-binding sites of BRCA1 and BRCA2 genes as risk factors for the development of early-onset and/or familial breast cancer. Asian Pac J Cancer Prev. 2014; 15:8319–8324. PMID:
25339023.

30. Cao J, Luo C, Yan R, Peng R, Wang K, Wang P, et al. rs15869 at miRNA binding site in BRCA2 is associated with breast cancer susceptibility. Med Oncol. 2016; 33:135. PMID:
27807724.

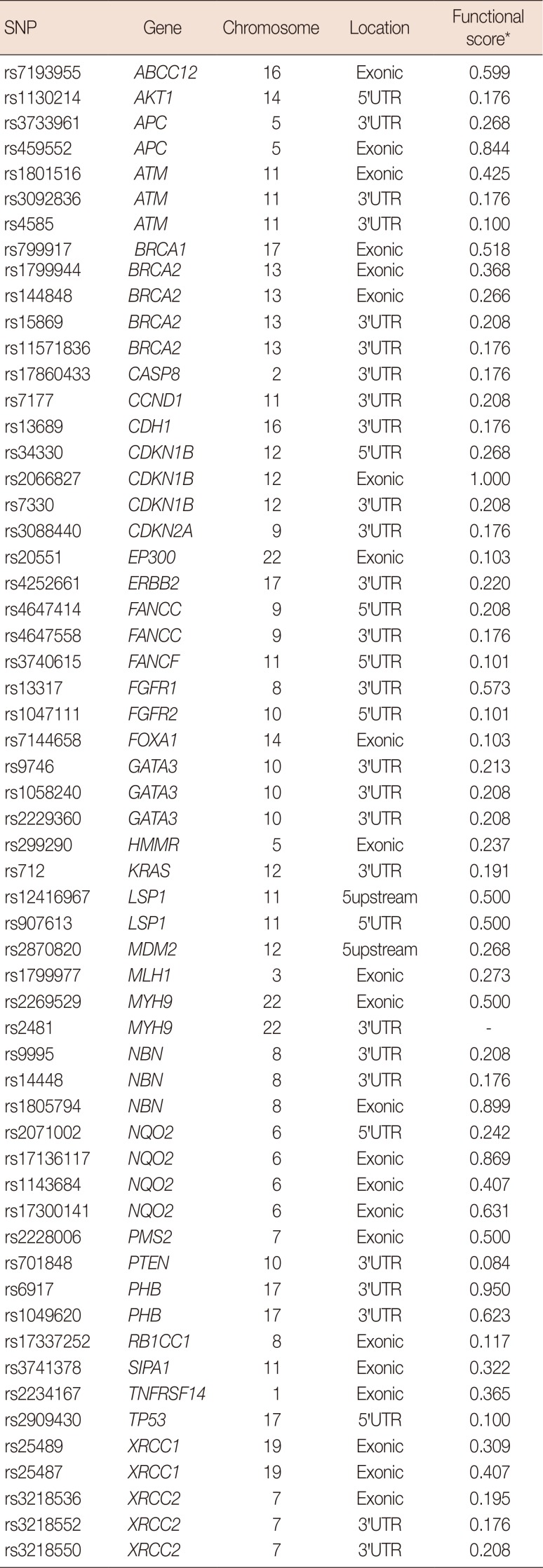
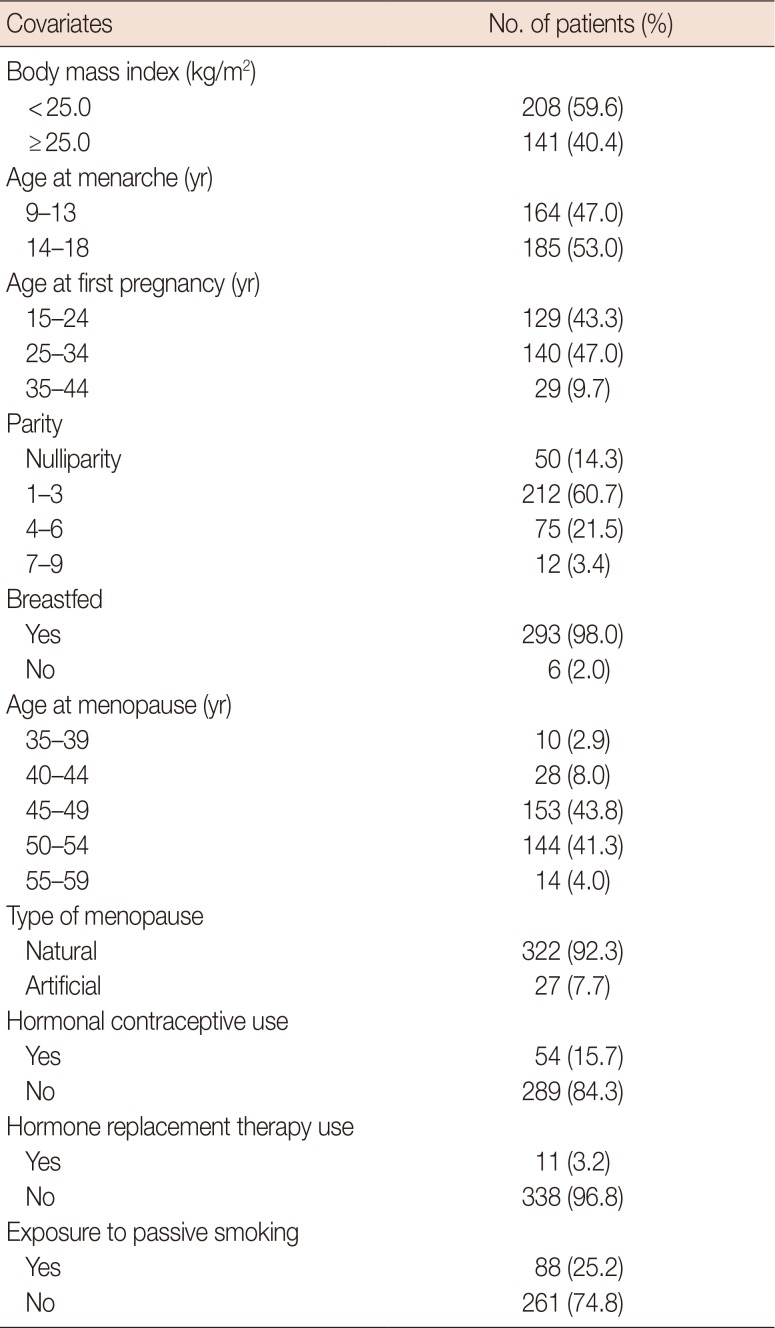
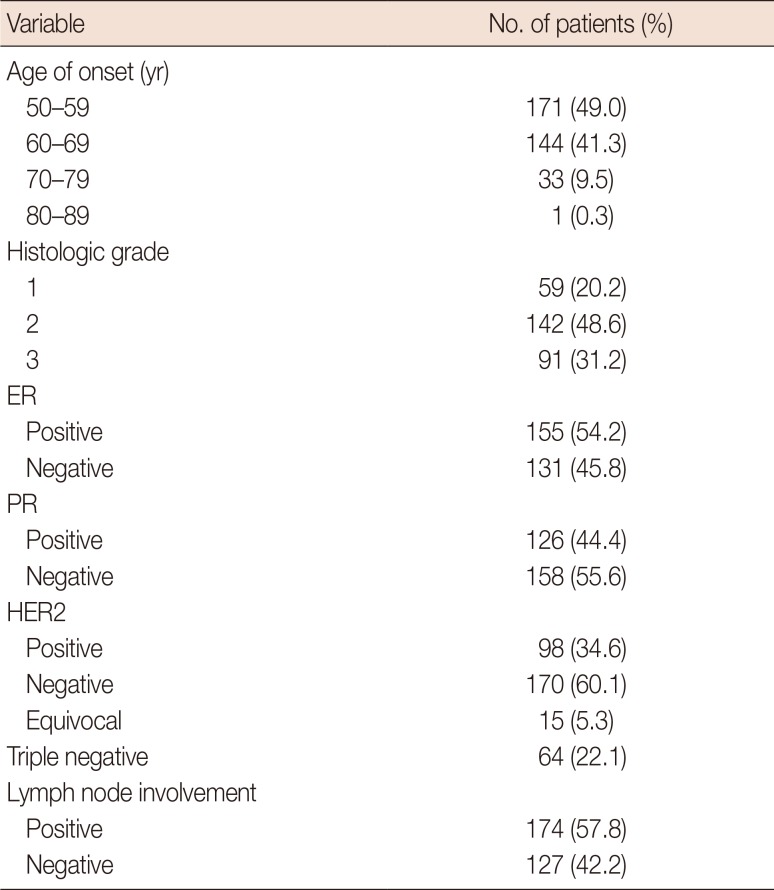
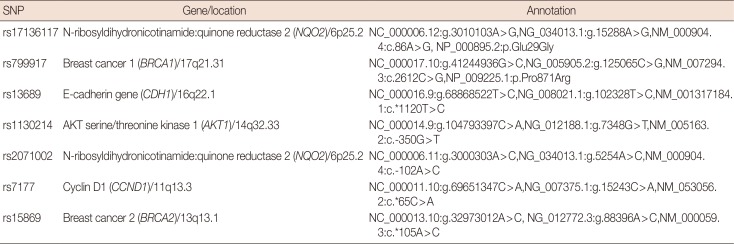




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download