Abstract
Purpose
The treatment of triple-negative breast cancer (TNBC) remains challenging, due to the absence of estrogen, progesterone, and human epidermal growth factor receptors. This study was designed to evaluate the efficiency and safety of cytokine-induced killer (CIK) cell immunotherapy, following regular chemotherapy, for patients with TNBC.
Methods
A total of 340 patients with postmastectomy TNBC, from January 1, 2010 to June 30, 2014, were included in this retrospective study. Seventy-seven patients received CIK cell immunotherapy, following regular chemotherapy (arm 1), and 263 patients received regular chemotherapy alone (arm 2). The primary aim was overall survival (OS) and disease-free survival (DFS), and the treatment responses and adverse events were also evaluated.
Results
The 5-year DFS and OS rates in arm 1 were 77.9% and 94.3%, compared with 69.8% and 85.6% in arm 2, respectively (p=0.159 and p=0.035, respectively). This clearly shows that there was no statistical difference in the 5-year DFS between the two groups. Multivariate analyses of arm 1 indicated that a Karnofsky performance score (KPS) ≥90 and stage I/IIA disease were significantly associated with a prolonged DFS period (hazard ratio [HR], 0.25; 95% confidence interval [CI], 0.09–0.74; p=0.012; and HR 0.21; 95% CI, 0.06–0.82; p=0.024, respectively), but a KPS ≥90 and stage I/IIA disease were not independent prognostic factors for OS. Toxicity was mild in patients who received the CIK therapy.
Breast cancer is one of the most common malignancies and the leading cause of cancer-related mortality among women globally [1]. Today, the incidence of breast cancer is still increasing from year to year, especially in developing countries [2]. However, with the emergence of targeted drugs, the efficiency of breast cancer treatment has improved, and the mortality rate of breast cancer has decreased in developed countries [3]. Triple-negative breast cancer (TNBC), which encompasses a significant subgroup that is defined by the absence of the estrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2), accounts for approximately 15% to 20% of breast cancer cases [4]. Based on previous observations, patients with TNBC present a heterogeneous disease with aggressive clinical behavior and adverse outcomes [56]. For instance, a study that was conducted by Dent et al. [5] on a large number of patients with TNBC from a single institution clearly showed that patients with TNBC have a shorter median time to death (4.2 years) and are more likely to experience distant recurrences, compared to patients with other breast cancers (33.9% vs. 20.4%). Due to the lack of a specific therapeutic target, patients with TNBC have gained little benefit from the currently available targeted treatments. Therefore, the currently used therapeutic regimen for patients with TNBC is still taxane- and anthracycline-based chemotherapy, according to the majority of national and international guidelines, but only a subset of the chemotherapy-sensitive patients benefit from this treatment [78]. Further efforts are needed to improve the current therapeutic modalities and explore novel therapies for TNBC, with the aim of improving patient care and increasing survival.
Recently, immunotherapy has become the fourth most important treatment for malignant tumors, ranked after surgery, radiotherapy, and chemotherapy, and has shown promising results [9]. Many adoptive immunotherapies, such as therapies using lymphokine-activated killer cells, tumor-infiltrating lymphocytes, cytotoxic T cells, and anti-CD3 monoclonal antibody-induced killer cells, have been reported in the past decades; however, due to low antitumor activities their therapeutic efficacy is limited [10]. The use of cytokine-induced killer (CIK) cells is a promising strategy for cancer therapy since CIK cells can proliferate rapidly in vitro, on induction by several cytokines [9]. Moreover, CIK cells are non-major histocompatibility complex-restricted cells that can express both T cell and natural killer cell markers, CD3 and CD56, respectively, which induce strong cytolytic activities against susceptible tumors [11]. In contrast, CIK cells can regulate and enhance immune functions in patients with cancer [12]. Based on the above advantages, CIK cells have shown excellent antitumor activities in hematological malignancies and various solid tumors, including gastric cancer, hepatocellular carcinoma, pancreatic cancer, lung cancer, colorectal cancer, ovarian cancer, and renal carcinoma [131415161718]. Till date, the therapeutic effects of CIK cells on TNBC, including preclinical and clinical studies, have rarely been reported. The purpose of this retrospective case-control study is to evaluate the clinical efficacy of CIK cell immunotherapy in patients with TNBC.
This study was approved by the State Food and Drug Administration of China (2006L01023) and by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (E2007006), according to the guidelines of the Declaration of Helsinki. All patients signed informed consent before entering the study.
Between January 1, 2010 and June 30, 2014, the medical records of patients with TNBC from the computerized database at the Tianjin Medical University Cancer Institute and Hospital were reviewed. This study enrolled a total of 340 patients with TNBC, including 77, who received CIK cell immunotherapy following regular chemotherapy, and 263, who received regular chemotherapy alone. All patients were required to meet the following conditions: pathology confirmed TNBC, wherein immunohistochemical staining was defined as follows: ER and PR nuclear staining <1%, ER- and PR-negative; and HER-2 staining 0 to 2+ by immunohistochemistry or nonamplified HER2 by fluorescence in situ hybridization, HER2-negative; age between 18 and 70 years; a Karnofsky performance score (KPS) >70%; and adequate bone marrow, renal, and liver functions. Patients were excluded if any of these criteria were not met, and patients with congestive heart failure, severe arrhythmia or severe coronary heart disease, human immunodeficiency virus infection, chronic progressive hepatitis, or peripheral neuropathy were also excluded, as were patients that were pregnant or lactating. The data from all of the patients are shown in Table 1.
After surgery, anthracycline-based (CEF: 5-FU 400 mg/m2; epirubicin 50 mg/m2; cyclophosphamide 500 mg/m2; cycled every 21 days), anthracycline- and taxane-based (TAC: docetaxel 75 mg/m2 or paclitaxel 150 mg/m2; doxorubicin 50 mg/m2; cyclophosphamide 600 mg/m2; cycled every 21 days), or taxane-based (TC: docetaxel 75 mg/m2 or paclitaxel 150 mg/m2; cyclophosphamide 600 mg/m2; cycled every 21 days) regimens were administered as the regular adjuvant chemotherapies. After several cycles of chemotherapy, all patients in arm 1 were given an infusion of CIK cells. Schmeel et al. [19] determined the number of CIK cells needed for a single infusion in 33 of 45 studies and found that the median and mean count of CIK cells were 5×109 and 7.7×109, respectively. In our treatments, we always maintained this standard. Some patients received neoadjuvant chemotherapy and subsequent radiotherapy because of the respective disease treatments of the two groups.
Autologous CIK cells were prepared, as described in our previous studies [15]. Briefly, peripheral blood mononuclear cells were collected from patients with TNBC using a Code Spectra Apheresis System (Caridian BCT, Lakewood, USA). Then, they were cultured in a medium containing 50 ng/mL anti-CD3 antibody (e-Bioscience, San Diego, USA), 100 U/mL recombinant human interleukin (IL)-1α, and 1,000 U/mL interferon-γ (IFN-γ), to induce CIK cells, at 37℃ with 5% CO2 for 24 hours. Subsequently, 300 U/mL of recombinant human IL-2 was added to the medium, and the medium was regularly replaced with fresh IFN-γ- and IL-2-containing medium every 5 days. All products were free of bacterial, fungal, and mycoplasma contamination and contained <5 endotoxin units. On day 14, the CIK cells were harvested, and the median number of CIK cells was 7.4×109 with a viability of greater than 95%. This method led to a significantly higher proportion of the CD3+CD56+ cellular subset.
Follow-up was completed for all the patients from the date of the initial treatment until June 1, 2017 or death. Response was defined according to the National Cancer Institute's Response Evaluation Criteria in Solid Tumors [20]. In this study, telephone consultations were conducted for each patient. Furthermore, we also reviewed patient records with respect to basic serum chemistry, chest X-ray, and ultrasound scans of the liver and abdomen. If the patients had a recurrence or metastasis during the follow-up period, remedial treatments, including surgery, chemotherapy, or radiation treatment, were recommended. Adverse events were evaluated according to the World Health Organization criteria.
Overall survival (OS) was defined as the period from the initial treatment until death. If the patient was still alive at the end of the follow-up period, the OS was defined from the date of initial treatment to the date of last contact. Disease-free survival (DFS) was calculated from the date of initial treatment to the date of first progression or date of last contact, and patients who were still alive were censored at the time of last contact. Differences in the demographic and clinical variables of the two groups, as well as their responses to therapy, were analyzed using the chi-square and Fisher exact tests. Distribution of the survival times and rates were determined by the Kaplan-Meier method. The Cox proportional hazard model was used for univariable and multivariable analyses. All calculations were performed using SPSS version 18.0 software (SPSS Inc., Chicago, USA), and differences with p-values of <0.05 were considered significant.
A total of 340 cases of TNBC were analyzed by collecting case and follow-up information, and the cases included 77 patients, who were treated with postoperative chemotherapy combined with CIK cell therapy, and 263 patients, who were treated with chemotherapy alone. The biological characteristics of the two groups are detailed in Table 1. There were no significant differences between the two groups (p>0.05).
The median follow-up time for the patients in arm 1 was 58.4 months (range, 21.5–88.9 months), and for the patients in arm 2, it was 53.5 months (range, 1.6–88.9 months). The 5-year OS rate for arm 1 was significantly higher than the rate for arm 2 (94.3% vs. 85.6%, p=0.035) (Figure 1A). The 5-year DFS rate for arm 1 was also superior to that of arm 2 (77.9% vs. 69.8%, p=0.159) (Figure 1B), with no statistical significance. At the end of the follow-up period, there were 16 patients (20.8%) in arm 1 and 76 patients (28.9%) in arm 2 who exhibited disease progression; among them, 15 patients (19.5%) in arm 1 and 63 patients (24.0%) in arm 2 had disease progression within three years, accounting for 93.8% and 82.9% of the patients with progressive disease in arms 1 and 2, respectively (p=0.412). A total of four (5.2%) deaths in arm 1 and 37 (14.1%) deaths in arm 2 were reported, and the patients who died within 3 years accounted for 50.0% and 83.8% of the overall deaths in arms 1 and 2, respectively (p=0.017).
In our subgroup analysis, CIK cell treatment prolonged the OS of patients with TNBC in the N1, N2, and N3 stages; however, similar results were not observed for the DFS (Figure 2A). Furthermore, we found that there were no significant differences between the DFS and OS of patients with TNBC stage N0 or TNM stage I, IIA, IIB, or III, between the two arms (Figure 2B-2D).
There were no differences in the local recurrence rates, regional metastases, and distant metastases between the two arms of the study (7.8% vs. 7.6%, p=0.957; 6.5% vs. 4.6%, p=0.494; and 13.0% vs. 16.3%, p=0.643, respectively). In our study, the frequent metastatic sites of TNBC were the bone, lung, liver, brain, and adrenal glands, the details of which are shown in Supplementary Table 1 (available online). The disease control rates at 3 years and 5 years were not significantly different between the two arms (80.5% vs. 76.0%, p=0.412 and 79.2% vs. 71.1%, p=0.177, respectively).
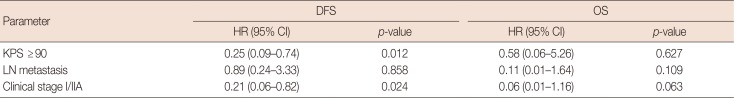
All patients in arm 1 received CIK cell treatment after chemotherapy, and the details are shown in Supplementary Table 2 (available online). The median CIK cell immunotherapy frequency was six cycles (range, 1–19 cycles), and we found that ≥6 cycles of CIK cell treatment was significantly associated with good prognoses (p=0.002 in DFS, p=0.024 in OS). In the univariate analysis, a KPS ≥90 was associated with better DFS, and the patients with early-stage disease had better DFS and OS than those with advanced-stage disease (Table 2). Moreover, in the multivariate analysis of the CIK cell therapy group, KPS ≥90 and patients who were clinical stage I/IIA had a better DFS, although these were not independent prognostic factors for OS (Table 3).
In our study, we observed high frequencies of chemotherapeutic toxicities that included transient fever, fatigue, nausea, vomiting, and anemia of grade 1–2, which were not significantly different in frequency between arm 1 (54 patients with grade 1–2 toxicity) and arm 2 (197 patients with grade 1–2 toxicity, p=0.402). There were 15 patients in arm 1 and 35 patients in arm 2 who presented with grade 3–4 myelosuppression after chemotherapy in this study (p=0.179). In arm 1, we did not observe any significant febrile symptoms in the 77 patients treated with CIK cells. Only six patients had mild liver dysfunction after transfusion of the CIK cells, and their liver functions returned to normal after symptomatic treatment. In general, no severe side effects were observed in the patients who received CIK cell therapy.
With poor prognosis, the treatment for TNBC remains a challenge in clinical practice. At present, anthracycline- and taxane-based chemotherapies are still the main therapeutic regimens, due to the lack of specific treatment targets. However, for most patients with TNBC, chemotherapy alone is associated with a high risk of recurrence, and as such, these patients have a high risk of relapse and experience a sharp decrease in survival three to five after treatment [5]. More than a decade of clinical studies have demonstrated that CIK treatment provides positive clinical efficacy in several types of cancers, indicating that immune-based therapy provides a promising therapeutic approach for patients with TNBC [21]. However, the research on CIK cell therapy in breast cancer, especially in TNBC, remains relatively limited. This retrospective study of CIK cells, combined with chemotherapy for the treatment of patients with TNBC, provides valuable clinical data, which will guide future research.
The positive effects of CIK cell therapy, in combination with chemotherapy, can probably be associated with their synergistic effects. Recently, some reports have found that CIK cells have intense tumor killing activity in vitro and in vivo against putative cancer stem cells that were resistant to chemotherapy [2223]. Furthermore, chemotherapy can regulate the immune status of patients with cancer [24]. Thus, combined therapy might be an optimized strategy to gain improved therapeutic efficacy in patients with TNBC.
Our results suggest that CIK adoptive immunotherapy, in combination with standard chemotherapy regimens, significantly improves the OS of patients with TNBC, and we observed that the DFS increased with the combined therapy, despite the lack of statistical difference. While there are some differences between our results and those of related TNBC studies, a phase II clinical trial in metastatic colorectal cancer showed the same outcome [15]. Importantly, CIK-associated toxicity was mild in our trial, which suggests that CIK cell treatment has better efficacy and safety in patients with postmastectomy TNBC. Our study also found that patients with early-stage disease had better DFS and OS than patients with IIB/III stage TNBC. We observed that CIK cell treatment prolonged the OS of patients with TNBC who were in stages N1, N2, and N3, although this result was not observed for patients with stage N0, I, IIA, IIB, or III disease [2526]. In our study, we found that the patients in the early stages received the greatest benefit from CIK cell immunotherapy, and this result is consistent with some previous studies [2728]. This may indicate that the immune function of patients with advancedstage TNBC is suppressed and that a high tumor burden influences the therapeutic effects of CIK cells [29]. We also observed that most patients exhibited metastasis or death within 3 years of diagnosis, which is consistent with the view that the risk of distant recurrence for patients with TNBC peaks at approximately 3 years [5]. We also observed a trend toward decreased mortality rates for patients with TNBC in arm 1 within 3 years of diagnosis.
There may be a few shortcomings of this study. First, this is a retrospective study, and all the patients were from our hospital. As such, our data may not completely reflect the status of patients in other hospitals. Moreover, family economic conditions and supportive care treatment might affect the survival differences between the two arms. Second, the use of chemotherapeutic agents was not standardized in this trial, but there were no differences between the CIK cell therapy group and the chemotherapy group (Supplementary Table 3, available online); therefore, we suspect that differences in the chemotherapy regimens would not significantly influence the OS. Thus, the therapeutic benefits that we observed in the combined treatment group were considered to result from the CIK cell transfusion.
In summary, this retrospective study revealed a relationship between CIK cell immunotherapy and TNBC prognosis. As tumor cells can experience immune escape through the evolution of poorly immunogenic tumor variants, immunosuppression, and immune system exhaustion [30], it is necessary to discover better therapies for targeting tumor cells and to further define the optimal combinations for treatment approaches for this unique breast cancer subtype.
References
1. Benson JR, Jatoi I. The global breast cancer burden. Future Oncol. 2012; 8:697–702. PMID: 22764767.

2. Guo P, Huang ZL, Yu P, Li K. Trends in cancer mortality in China: an update. Ann Oncol. 2012; 23:2755–2762. PMID: 22492700.

3. Clarke M. Meta-analyses of adjuvant therapies for women with early breast cancer: the Early Breast Cancer Trialists' Collaborative Group overview. Ann Oncol. 2006; 17(Suppl 10):x59–x62. PMID: 17018753.

4. Mouh FZ, Mzibri ME, Slaoui M, Amrani M. Recent progress in triple negative breast cancer research. Asian Pac J Cancer Prev. 2016; 17:1595–1608. PMID: 27221827.

5. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13(15 Pt 1):4429–4434. PMID: 17671126.

6. Lara-Medina F, Pérez-Sánchez V, Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011; 117:3658–3669. PMID: 21387260.
7. Joensuu H, Gligorov J. Adjuvant treatments for triple-negative breast cancers. Ann Oncol. 2012; 23(Suppl 6):vi40–vi45. PMID: 23012301.

8. Schwentner L, Wolters R, Koretz K, Wischnewsky MB, Kreienberg R, Rottscholl R, et al. Triple-negative breast cancer: the impact of guideline-adherent adjuvant treatment on survival: a retrospective multicentre cohort study. Breast Cancer Res Treat. 2012; 132:1073–1080. PMID: 22205141.
9. Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2011; 137:305–310. PMID: 20407789.

10. Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011; 17:6287–6297. PMID: 21844012.

11. Yu R, Yang B, Chi X, Cai L, Liu C, Yang L, et al. Efficacy of cytokine-induced killer cell infusion as an adjuvant immunotherapy for hepatocellular carcinoma: a systematic review and meta-analysis. Drug Des Devel Ther. 2017; 11:851–864.

12. Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993; 21:1673–1679. PMID: 7694868.
13. Schmeel FC, Schmeel LC, Gast SM, Schmidt-Wolf IG. Adoptive immunotherapy strategies with cytokine-induced killer (CIK) cells in the treatment of hematological malignancies. Int J Mol Sci. 2014; 15:14632–14648. PMID: 25196601.
14. Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res. 2012; 18:1751–1759. PMID: 22275504.

15. Zhao H, Wang Y, Yu J, Wei F, Cao S, Zhang X, et al. Autologous cytokine-induced killer cells improves overall survival of metastatic colorectal cancer patients: results from a phase ii clinical trial. Clin Colorectal Cancer. 2016; 15:228–235. PMID: 27052743.

16. Wang Z, Liu Y, Li R, Shang Y, Zhang Y, Zhao L, et al. Autologous cytokine-induced killer cell transfusion increases overall survival in advanced pancreatic cancer. J Hematol Oncol. 2016; 9:6. PMID: 26842696.

17. Li R, Wang C, Liu L, Du C, Cao S, Yu J, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012; 61:2125–2133. PMID: 22581306.

18. Liu J, Li H, Cao S, Zhang X, Yu J, Qi J, et al. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. J Immunother. 2014; 37:115–122. PMID: 24509174.

19. Schmeel LC, Schmeel FC, Coch C, Schmidt-Wolf IG. Cytokine-induced killer (CIK) cells in cancer immunotherapy: report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2015; 141:839–849. PMID: 25381063.

20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.

21. Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013; 5:169–181. PMID: 23634195.

22. Gammaitoni L, Giraudo L, Leuci V, Todorovic M, Mesiano G, Picciotto F, et al. Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features. Clin Cancer Res. 2013; 19:4347–4358. PMID: 23794732.

23. Sangiolo D, Mesiano G, Gammaitoni L, Leuci V, Todorovic M, Giraudo L, et al. Cytokine-induced killer cells eradicate bone and soft-tissue sarcomas. Cancer Res. 2014; 74:119–129. PMID: 24356422.

24. Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010; 120:1111–1124. PMID: 20234093.

25. Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ, et al. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res. 2014; 20:3003–3011. PMID: 24668644.

26. Ho AY, Gupta G, King TA, Perez CA, Patil SM, Rogers KH, et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer. 2012; 118:4944–4952. PMID: 22392492.

27. Li H, Huang L, Liu L, Wang X, Zhang Z, Yue D, et al. Selective effect of cytokine-induced killer cells on survival of patients with early-stage melanoma. Cancer Immunol Immunother. 2017; 66:299–308. PMID: 27889798.

28. Li DP, Li W, Feng J, Chen K, Tao M. Adjuvant chemotherapy with sequential cytokine-induced killer (CIK) cells in stage IB non-small cell lung cancer. Oncol Res. 2015; 22:67–74. PMID: 25706393.

29. Fichtner S, Hose D, Engelhardt M, Meißner T, Neuber B, Krasniqi F, et al. Association of antigen-specific T-cell responses with antigen expression and immunoparalysis in multiple myeloma. Clin Cancer Res. 2015; 21:1712–1721. PMID: 25609061.

30. Mata-Molanes JJ, Sureda González M, Valenzuela Jiménez B, Martínez Navarro EM, Brugarolas Masllorens A. Cancer immunotherapy with cytokine-induced killer cells. Target Oncol. 2017; 12:289–299. PMID: 28474278.

SUPPLEMENTARY MATERIALS
Supplementary Table 3
Distribution of chemotherapy regimens of patients in the two arms
Figure 1
Overall survival (OS) and disease-free survival (DFS) of patients in arm 1 and arm 2. Kaplan-Meier curves for OS (A) and DFS (B) was used to compare the survival rates in patients of arm 1 and arm 2.
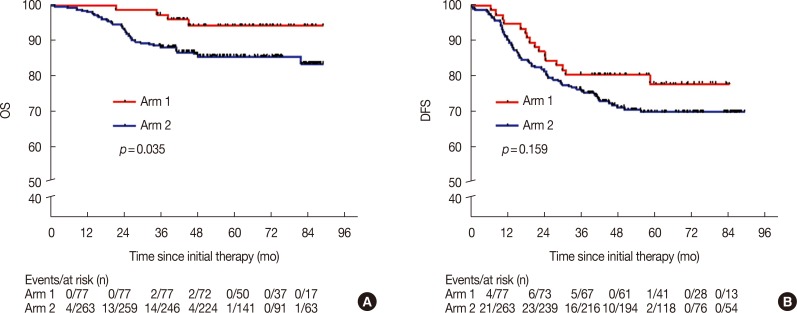
Figure 2
Subgroup analysis to estimate the benefits of cytokine-induced killer treatment. (A) N1,2,3 stages. (B) N0 stage. (C) I and IIA stages. (D) IIB and III stages. Left, disease-free survival (DFS) curves; right, overall survival (OS) curves.
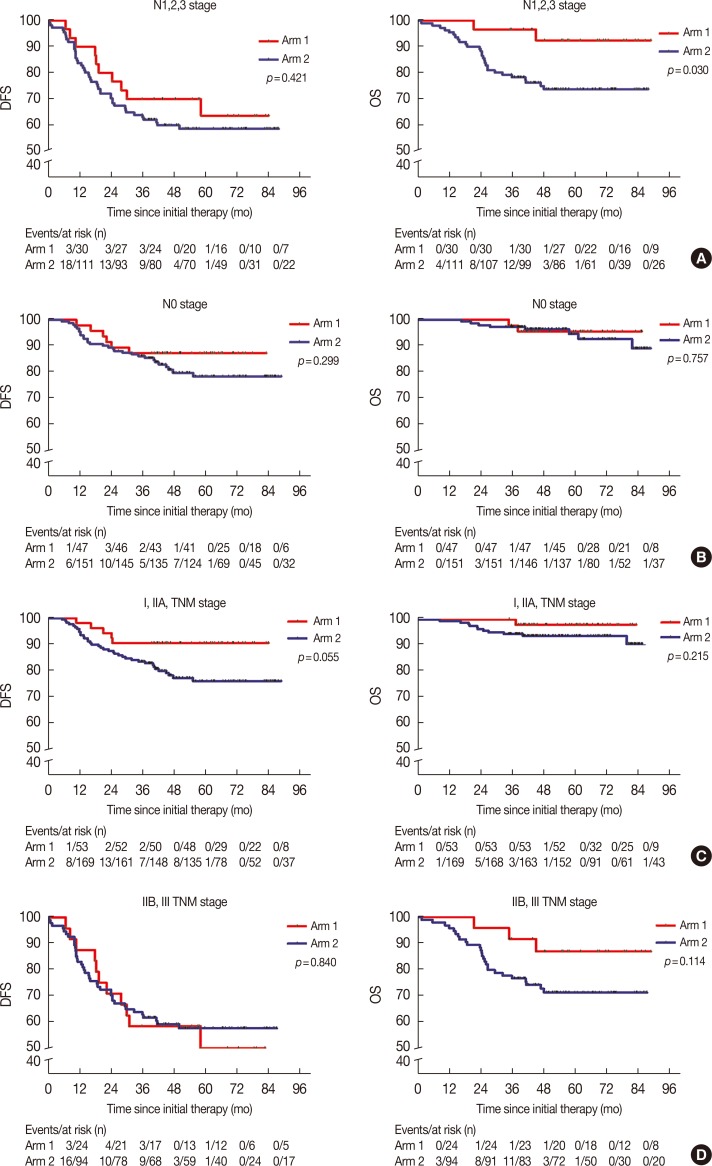
Table 1
Distribution of demographic and clinical characteristics of patients in the two groups
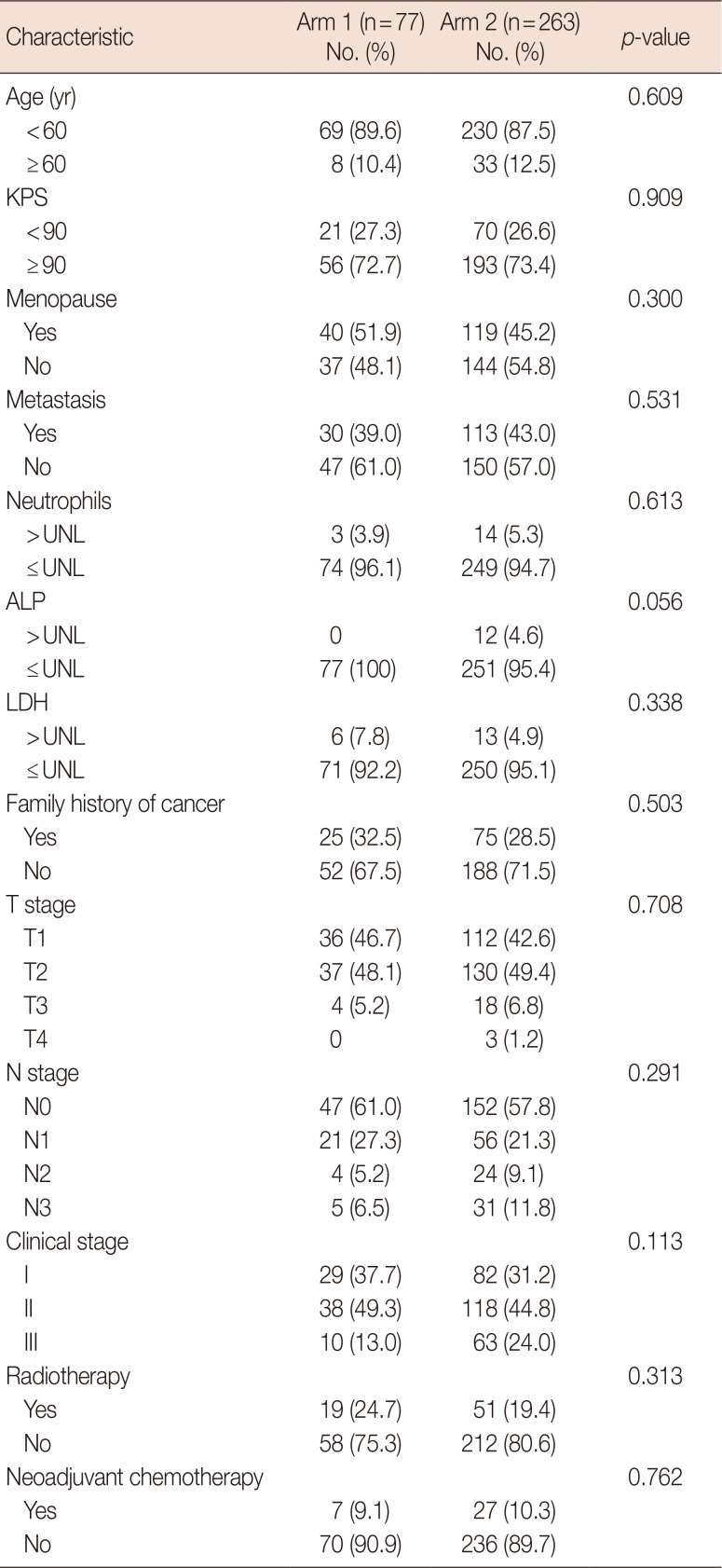
Table 2
Univariate analysis of 77 patients' demographic and clinical characteristics and survival in arm 1
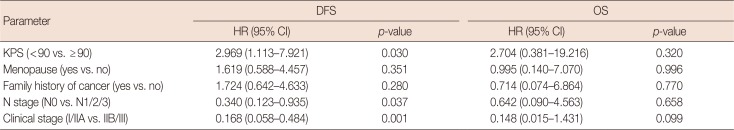
Table 3
Multivariate analysis of 77 patients' demographic and clinical characteristics and survival in arm 1




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download