Nature harbors different classes of fungi, some of which are pathogenic for humans.
Aspergillus is a genus of fungi belonging to the Ascomycota phylum. Despite the enormous diversity among species of
Aspergillus, only a few thermotolerant groups are capable of causing an opportunistic infection, known as aspergillosis, in human beings.
123 Aspergilloma is the most common subtype of aspergillosis; it is defined as noninvasive chronic fungal sinusitis, and is predominantly seen in the maxillary antrum of immunocompetent hosts.
4 Aspergilloma is usually asymptomatic and it may take several years for symptoms to occur.
5 It usually affects a unilateral sinus cavity and is detected incidentally, with the characteristic appearance of an area with iron-like density resembling a foreign body in a homogenously clouded maxillary sinus on radiographic examination.
46 In symptomatic cases, the clinical manifestation of aspergilloma is often nonspecific and includes purulent or blood-stained nasal discharge, chronic sinus pain, nasal congestion, impaired sense of smell, headache, and orbicular pain.
7 Complete removal of the lesion via the Caldwell-Luc or endoscopic surgical techniques, with the establishment of natural sinus drainage, is sufficient for the management of aspergilloma, and leads to a low recurrence rate.
478
The aim of this paper is to discuss the etiology and management of maxillary sinus aspergilloma of odontogenic origin, to present 2 new cases, and to review the previously reported cases. For this purpose, a systematic search of the literature on maxillary sinus aspergilloma of odontogenic origin was carried out on PubMed/MEDLINE and Google Scholar through 2017. The search was performed using the keywords ‘aspergillosis,’ ‘aspergillus,’ ‘aspergilloma,’ ‘fungus ball,’ ‘mycetoma,’ ‘maxillary sinus,’ ‘odontogenic,’ and ‘dental’ and combinations thereof.
Discussion
Fungal infection of the maxillary sinus is relatively rare in healthy individuals, but because of the globally uncontrolled consumption of chemotherapeutics that cause patients to be vulnerable to fungal infections and as a result of improvements in diagnostic imaging techniques, the detection of this infection among healthy subjects seems to be increasing.
47 It has been reported that more than 10% of patients who had chronic sinusitis were found to have aspergilloma, predominantly in the maxillary sinus.
58 The main aspects of the pathophysiology of aspergilloma of the maxillary sinus are still debated, although contamination of the maxillary sinus with
Aspergillus has been suggested to occur through various pathways.
3 Because
Aspergillus species do not have the ability to penetrate intact mucus membranes,
Aspergillus is usually considered to cause infections in maxillary sinus as a result of the inhalation of the airborne spores of
Aspergillus that are ubiquitous in the environment.
13 According to the aerogenic theory, the accumulation of fungal spores in the maxillary sinus may become pathogenic under relatively anaerobic conditions.
9 However, unlike the other paranasal sinuses,
Aspergillus spores may also be transmitted to the maxillary sinus through an iatrogenic pathway associated with dental procedures.
110
Recently, an increasing number of researchers have suggested that because of the close relationship between the antral teeth and sinus floor, dentogenic factors increase the risk of aspergilloma of the maxillary sinus.
911 Tomazic et al.
9 suggested that if a dentogenic factor is present, the risk of developing an aspergilloma is 2.7-fold higher than in unaffected sinuses. Dental procedures are thought to be able to cause massive fungal inoculation of the maxillary antrum as a result of perforation of the sinus membrane.
8 Additionally, dental materials that contain heavy metals (e.g., zinc), such as root canal sealer, gutta-percha, silver cones, and amalgam, may penetrate into the sinus during dental procedures and provide favorable conditions for the growth of
Aspergillus species.
411 Although eugenol in dental materials has a fungicidal effect, it loses its inhibitory function when it penetrates into the sinus, enabling heavy metals to promote fungal growth.
12 Furthermore, dental procedures that perforate the sinus membrane can cause mucociliary paralysis and mucosal hyperemia, resulting in epithelial dysfunction in the maxillary sinus.
6 Due to disturbances in mucociliary action, the natural sinus drainage deteriorates and an anaerobic environment associated with local tissue hypoxia occurs.
8
Among the published cases of aspergilloma associated with dental procedures, the predominant etiologic factor is root canal treatment, but aspergilloma associated with dental implants, extraction, or grafting procedures has also been reported.
45710 In the studies of Tomazic et al.
9 and Legent et al.,
13 it was reported that 84% and 96% of patients with aspergilloma had undergone previous root canal therapy, respectively. Similarly, several cases of aspergilloma in the literature have been detected in sinuses that had been perforated by a previous dental procedure, while the contralateral side remained unaffected (
Table 1).
13456781011121415161718192021222324 Root canal treatment and the displaced root were considered to be etiologic factors for the occurrence of aspergilloma in our cases.
From a clinical point of view, aspergilloma is usually underestimated because the infection only becomes symptomatic after a long period of fungal contamination.
5 It was reported that in 29% of patients, aspergilloma was diagnosed 1 year after the onset of symptoms because of the noninvasive character and slow progression of the lesion.
14 Giardino et al.
15 reported a case of aspergilloma that arose 2 years after root canal therapy. In another case, Sohn et al.
7 reported a case of
Aspergillus 1 year after the patient had undergone sinus bone grafting. However, in some cases, the time of onset of the infection was shorter.
6 In our cases, the fungal infections were detected 6 and 13 years after the dental procedures, respectively. Thus, it is critical to ensure adequate follow-up after dental treatment involving the maxillary sinus.
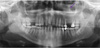
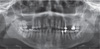
Panoramic radiographic examinations are a straightforward way to evaluate the maxillary sinus bilaterally for the diagnosis of aspergilloma.
14 Maxillary sinus aspergilloma is usually seen unilaterally, and bilateral lesions are very rare.
14 However, a case of bilateral maxillary-ethmoidal sinus aspergilloma that occurred after bilateral endodontic treatment was reported by Vinciguerra et al.
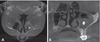
16 The pathognomonic iron-like density could be seen incidentally on panoramic radiography or on the Waters view.
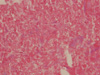
47 This characteristic appearance is due to high levels of calcium phosphate in the intracellular milieu of the necrotizing
Aspergillus cells, and in some cases may result from accumulation of the heavy metals that were pushed into the sinus with the dental materials.
7810 A more precise examination with computed tomography (CT) may be necessary to exclude other sinus diseases, such as antrolith, osteoma, mucocele, B cell lymphoma, squamous cell carcinoma, adenoid cystic carcinoma, and inflammatory myofibroblastic tumors, from the differential diagnosis.
14 The extent of the lesion, bone involvement, and erosion can also be evaluated using CBCT, which requires a lower radiation dose, is cost-effective, and is not time-consuming.
14 Magnetic resonance imaging (MRI) can also be helpful, as decreased signal intensity on T2-weighted MRI has been described as characteristic of aspergilloma, and MRI can also help clinicians to differentiate aspergilloma from inflammatory or neoplastic changes.
110
The treatment of aspergilloma primarily consists of surgical removal of the lesion. Both the Caldwell-Luc and endoscopic techniques can be used.
48 In most of the reported cases, the Caldwell-Luc procedure was used successfully for the management of aspergilloma.
510121417 Systemic antifungal therapy is not generally required. However, if symptoms persist for a long time after surgery, an oral antimycotic drug may be required as an additional therapy.
7 Nonetheless, clinicians should be careful about using these drugs because of severe adverse effects, such as nephrotoxicity.
6 Since bacterial superinfection can cause acute sinusitis attacks, an appropriate antibiotic therapy is recommended in order to avoid bacterial coinfections.
1
Different types of dental procedures that involve the maxillary sinus may facilitate the occurrence of fungal sinusitis, which shares similar features with other infections of the sinus. Clinicians should be aware of the possibility of fungal etiology, especially in cases resistant to treatment, and should follow the patient periodically if sinus perforation occurs during a procedure to minimize toxicity, costs, and other complications because of an inappropriate treatment strategy. Although the management of aspergilloma is much simpler than the management of the invasive form of aspergillosis, delays can occur in management because the likelihood of fungal origin may be underestimated. Thus, diagnostic tools, especially imaging modalities, play a crucial role in detecting aspergilloma, which is usually an incidental finding. CBCT can provide useful information to clinicians about the location and the extent of the lesion.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download