Abstract
Cemento-osseous dysplasia (COD) is a benign fibro-osseous lesion of bone, in which normal bone is replaced by fibrous tissue, followed by calcification with osseous and cementum-like tissue. COD is classified into 3 categories according to its location: periapical, focal, and florid COD (FCOD). On radiography, FCOD appears radiolucent in its early stages. As it matures, radiopacities appear within the lesion, causing them to show a mixed appearance of radiolucency and radiopacity. Because FCOD is usually asymptomatic and grows in a self-limited manner, it does not require treatment. Secondary infection is the most frequent cause of symptomatic cases. We report a case of FCOD with symptoms that appeared after a dental restoration procedure and persisted after repeated operations. The purpose of this report is to emphasize the importance of thorough radiological evaluations of patients with FCOD before treatment.
Cemento-osseous dysplasia (COD) is a group of disorders involving benign fibro-osseous lesions of bone. COD has a sexual and racial predilection, with women of African or southeastern Asian descent being most frequently affected, and the mean age at diagnosis is between 30 and 50 years. The reason for this racial predisposition remains unclear.12345
COD is a controversial term. Recently, the fourth edition of the World Health Organization Classification of Head and Neck Tumors reverted to the terminology “cemento- osseous dysplasia” from “osseous dysplasia” in order to recognize these tumors as odontogenic, with their origin in the periodontal ligament.6 Although there is little pathological or radiological rationale for the periodontal origin of these lesions, there is little doubt that COD is linked to the presence of teeth.7 Most lesions appear above the mandibular canal or below the junction of the hard palate, and thus are confined to alveolar bone. In addition, COD is more common in the mandible than in the maxilla.124
COD is classified into 3 categories according to its location; periapical COD (in the periapical region of the anterior teeth), focal COD (associated with a single tooth), and florid COD (FCOD). In FCOD, lesions appear in more than 1 quadrant.56
FCOD appears to represent a variant of the same disease process as COD, in which periapical bone is first replaced by fibrous tissue, followed by calcification with osseous and cementum-like avascular tissue.1247 On radiography, the lesions appear radiolucent in their early stage, but as they mature, radiopacities are formed within the lesions, causing them to show a mixed appearance of radiolucency and radiopacity. As a result, diffuse sclerosis, referred to as a cotton-wool appearance with a radiolucent halo, appears on radiographs.78
FCOD is usually asymptomatic and the adjacent teeth remain vital. The overlying gingiva also remains unaffected by the lesion. Unless FCOD is secondarily infected, it rarely causes clinical discomfort. However, contact of a lesion with oral flora can lead to infection, and sclerotic lesions are more vulnerable to infection.3 As these lesions have self-limited growth potential, asymptomatic lesions do not require treatment. However, if an invasive procedure, such as a tooth extraction, is absolutely necessary, it must be done with caution not to cause infection of the bone.910 This report describes a case of postoperative infected FCOD that became symptomatic and needed additional treatment.
A 40-year-old woman was referred to Chonbuk National University Dental Hospital for evaluation of pain in the tooth on chewing. The symptom had begun 3 months after restoration of the lower right first molar with a metal crown at a local dental clinic. The patient had no systemic disease and the extraoral examination was within normal limits. Intraoral examination revealed a metal crown on the right mandibular first molar, and the overlying gingiva and mucosa were normal, with no clinical signs of inflammation. The pulp vitality test in the right mandibular first molar was not performed due to the metal crown.
On a panoramic radiograph, multiple radiopaque masses surrounded by radiolucent halos were observed in the apical regions of the lower right first, second, and third molars and the lower left second molar. All the lesions were separated from the adjacent teeth by a radiolucent line without root resorption or displacement. Another completely radiopaque lesion was observed in the apical area of the right maxillary first molar (Fig. 1).
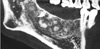
Cone-beam computed tomography (CBCT) with a small field of view for further investigation showed the lesions more clearly. The lesions were non-homogenous sclerotic masses with radiolucent rims, and were involved with the apices of the right mandibular second premolar, the first and second molars, the left mandibular second premolar, and the first and second molars. All the lesions were separated from the adjacent teeth by a radiolucent line without root resorption or displacement. Of particular note, diffuse sclerosis had progressed around the lesion at the lower left first molar. The lesions at the left mandibular first molar and the right mandibular first molar had invaded the cortical bone without expansion or perforation (Fig. 2).
A Tc-99m MDP scintigraph demonstrated high uptake in the posterior maxilla and mandible, suggesting that the lesions were growing, and the uptake was higher in the right mandible (Fig. 3).
Based on the clinical examination and radiographic evaluation, a diagnosis of mixed and end stage of FCOD was made. Because the patient's clinical symptoms persisted for more than 3 months and the patient continued to complain of discomfort, some mandibular lesions, including those near the right mandibular first and third molars and the left mandibular first molar, were removed. Postoperative histopathologic findings revealed multiple small fragments without any fibrous capsules. Multiple areas of irregular woven bone were distributed in dense fibrous tissue with no signs of inflammation (Fig. 4). A definitive diagnosis of FCOD was established based on the histopathologic examination.
On periodic radiographic examinations performed every 3 months after surgery, the remaining lesions adjacent to the right mandibular first molar that were not totally removed gradually grew. The apical lesion at the right mandibular second molar, which had not been removed, also showed a growth pattern in comparison to its previous appearance, displacing the mandibular canal downward. During the third year of follow-up, these lesions combined, accompanied by perforation of the lingual cortical plate. Similar findings were observed in the left mandible (Figs. 5 and 6).
The patient underwent a re-operation for the right posterior mandible because her clinical symptoms persisted. All the affected bone in the right mandible, including the right mandibular second premolar and the second molar, was removed (Fig. 7). As the left mandibular region remained asymptomatic, that area was not included in the operation.
A postoperative histopathologic examination showed numerous areas of woven bone in fibrous connective tissue with infiltration of inflammatory cells, implying secondary infection of the lesion (Fig. 8).
During periodic follow-up that extended for 18 months after secondary surgery, sclerotic masses surrounded by radiolucent halos appeared again at the previous surgical site, suggesting recurrence of the lesion (Figs. 9 and 10). The clinical symptoms had slightly subsided in comparison with the patient's previous state, but intermittent pain persisted in the right mandible.
FCOD is most commonly seen in Africans, followed by Asians and Caucasians. It also occurs frequently in middle-aged women.2 The reason for this racial and gender predilection is unknown. However, because race, sex, and age are important factors for the diagnosis of this disease, they must be considered when lesions suspicious for FCOD are observed in middle-aged Asian women, as in this case. Some cases have been reported to show a family history, but most cases appear to represent isolated instances.910111213141516 In this case, the patient's family history was not investigated.
Kawai et al.17 reported several radiographic patterns of COD: a well-defined radiolucency superimposed over apical areas of an adjacent tooth, a few calcified bodies seen in a radiolucent lesion, a central calcified mass in a radiolucent lesion, lobular or spherical calcified masses surrounded by radiolucent rims, irregularly shaped radiopacities around teeth in multiplex form, and periapical radiopacity of irregularly thickened root apices surrounded by radiolucency. These findings are also observed in FCOD. The radiological presentation of the lesions varies depending on their stage of maturation.11718 Moreover, as in this case, various stages of FCOD can appear simultaneously in a single patient.
FCOD is found in multiple quadrants and shows a marked tendency for symmetry. Symmetry with regard to the affected sextants, rather than mirror-image symmetry, has been reported to be an important feature of FCOD.219 In this case, the lesions were observed in 4 quadrants, including the left posterior maxilla, as observed during follow-up, and showed symmetry in the sextants.
The initial radiographic findings of FCOD are similar to those of periapical inflammatory lesions, which can result in unnecessary treatment of teeth, such as root canal treatment or extraction. Careful assessment of the radiographs can help to avoid unnecessary invasive treatment. FCOD can be differentiated from periapical inflammatory lesions by its typical radiographic appearance of multiple and symmetrical lesions with a mixed radiolucent and radiopaque pattern. The pulp vitality test is also crucial for the differential diagnosis of FCOD.1117202122
Generally, FCOD is symptom-free unless secondarily infected. Pain has been reported to be the most frequent symptom. Many studies in the literature have reported cases of FCOD complicated by infection from pulpal disease, periodontitis, or exposure of the lesion to oral flora.212212324 Secondary infection can be caused by improper treatment approaches, such as endodontic treatment, biopsy, extraction of teeth, and excision of the lesion. Complications can also occur under a denture in the affected area, as the result of progressive alveolar atrophy. Once symptoms appear, further interventions are required. In this case, pain began after caries removal and restoration in the lower right first molar. The pain lasted for 3 months and persisted after resection of the lesion. The best management in such circumstances is regular follow-up with radiographic examinations to observe healing or recurrence.
Recently, interest in FCOD, including other fibro-osseous lesions, has been increasing in association with the wider use of dental implants because of concerns about whether implantation can cause pathologic changes in the lesion.2526 Several cases have reported successful and failed implantation in patients with FCOD and other fibro-osseous lesions.182728 The quality of the alveolar bone is an important factor in the decision to perform implantation and in its success rate. If implants are needed at the site of the lesion, regions with higher radiopacity should be avoided, because the hypovascularity of such regions makes them more susceptible to infections that could cause implant failure.1828
As discussed above, radiology is central for the diagnosis and follow-up management of FCOD. However, due to the multiplicity of the lesions, mistakes may be made in the radiologic diagnosis depending on the imaging method. Periapical radiography of only some areas is not sufficient to diagnose all FCOD lesions present in a given case. It is important to evaluate the entire jaw, at least with a full-mouth series or panoramic radiograph, and CBCT may also be required.
In conclusion, FCOD occurs symmetrically in the apical region of the jaw bones, and shows various radiological features depending on its stage of maturation. The initial lesion is often misdiagnosed as a periapical inflammatory lesion, and the symptoms associated with FCOD are usually caused by secondary infection of the lesion. Therefore, clinicians should be careful not to cause secondary infection via unnecessary treatment. Radiographs play a key role in the diagnosis and treatment of patients with FCOD. For dental treatments such as tooth extraction or implants, clinicians should make a diagnosis and treatment plan based on an in-depth radiological evaluation.
Figures and Tables
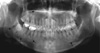 | Fig. 1Panoramic image shows multiple sclerotic masses with radiolucent rims in the apical region of the left mandibular second molar and the right mandibular first, second, and third molars (arrows), as well as completely radiopaque masses in the apical region of the right maxillary first molar and the left maxillary second premolar (arrowhead). |
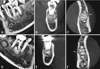 | Fig. 2Cone-beam computed tomography scans reveal well-defined radiopaque masses surrounded by radiolucent rims in the periapical regions of the left mandibular second premolar and first molar (A–C) and the right mandibular first and second molar (D–F). All the lesions were separated from the adjacent teeth by a radiolucent line. Invasion of the cortical bone can be observed (arrows). (A and D: sagittal, B and E: frontal, C and F: axial scan) |
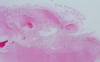 | Fig. 4Histopathologic examination shows multiple areas of irregular woven bone in dense fibrous stroma without any fibrous capsules, representing cemento-osseous dysplasia (H&E stain, original magnification ×100). |
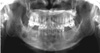 | Fig. 5Panoramic image 3 years after surgery shows the remaining calcified masses around the right mandibular first molar and increased periapical radiolucency at the right mandibular second molar. |
 | Fig. 6A and B. Cone-beam computed tomography (CBCT) scans 3 years after surgery reveal the remaining and newly-formed radiopacities inside the lesion. C. Axial CBCT scan shows perforation of the lingual cortical plate near the right mandibular second molar. |
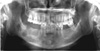 | Fig. 7Panoramic image obtained immediately after secondary surgery shows that all the lesions of the right mandible had been removed. |
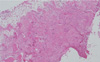 | Fig. 8Histopathologic finding shows numerous areas of woven bone in fibrous connective tissue with infiltration of inflammatory cells (H&E stain, original magnification ×40). |
References
2. MacDonald-Jankowski DS. Florid cemento-osseous dysplasia: a systematic review. Dentomaxillofac Radiol. 2003; 32:141–149.

3. Mainville GN, Turgeon DP, Kauzman A. Diagnosis and management of benign fibro-osseous lesions of the jaws: a current review for the dental clinician. Oral Dis. 2017; 23:440–450.

4. Su L, Weathers DR, Waldron CA. Distinguishing features of focal cemento-osseous dysplasia and cemento-ossifying fibromas. II. A clinical and radiologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997; 84:540–549.
5. Cavalcanti PH, Nascimento EH, Pontual ML, Pontual AD, Marcelos PG, Perez DE, et al. Cemento-osseous dysplasias: imaging features based on cone beam computed tomography scans. Braz Dent J. 2018; 29:99–104.

6. El-Mofty SK, Nelson B, Toyosawa S, Wright JM. Cementoosseous dysplasia. In : El-Naggar AK, Chan JK, Rubin Grandis J, Takata T, Slootweg PJ, editors. International Agency for Research on Cancer. WHO classification of head and neck tumours. 4th ed. Lyon: International Agency for Research on Cancer;2017. p. 254–255.
8. Mortazavi H, Baharvand M, Rahmani S, Jafari S, Parvaei P. Radiolucent rim as a possible diagnostic aid for differentiating jaw lesions. Imaging Sci Dent. 2015; 45:253–261.

9. Singer SR, Mupparapu M, Rinaggio J. Florid cemento-osseous dysplasia and chronic diffuse osteomyelitis Report of a simultaneous presentation and review of the literature. J Am Dent Assoc. 2005; 136:927–931.
10. Cavalcante MB, de Oliveira Lima AL, Júnior MA, Santos MB. Florid cemento-osseous dysplasia simultaneous the chronic suppurative osteomyelitis in mandible. J Craniofac Surg. 2016; 27:2173–2176.

11. Delai D, Bernardi A, Felippe GS, da Silveira Teixeira C, Felippe WT, Santos Felippe MC. Florid cemento-osseous dysplasia: a case of misdiagnosis. J Endod. 2015; 41:1923–1926.

12. Dağistan S, Tozoğlu Ü, Göregen M, Çakur B. Florid cemento-osseous dysplasia: a case report. Med Oral Patol Oral Cir Bucal. 2007; 12:E348–E350.
13. Wakasa T, Kawai N, Aiga H, Kishi K. Management of florid cemento-osseous dysplasia of the mandible producing solitary bone cyst: report of a case. J Oral Maxillofac Surg. 2002; 60:832–835.

14. Bencharit S, Schardt-Sacco D, Zuniga JR, Minsley GE. Surgical and prosthodontic rehabilitation for a patient with aggressive florid cemento-osseous dysplasia: a clinical report. J Prosthet Dent. 2003; 90:220–224.

15. Toffanin A, Benetti R, Manconi R. Familial florid cemento-osseous dysplasia: a case report. J Oral Maxillofac Surg. 2000; 58:1440–1446.

16. Önder B, Kurşun Ş, Öztaş B, Barş E, Erdem E. Florid osseous dysplasia in a middle-aged Turkish woman: a case report. Imaging Sci Dent. 2013; 43:197–200.

17. Kawai T, Hiranuma H, Kishino M, Jikko A, Sakuda M. Cemento-osseous dysplasia of the jaws in 54 Japanese patients: a radiographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 87:107–114.
18. Esfahanizadeh N, Yousefi H. Successful implant placement in a case of florid cemento-osseous dysplasia: a case report and literature review. J Oral Implantol. (in press).

19. Kim JH, Song BC, Kim SH, Park YS. Clinical, radiographic, and histological findings of florid cemento-osseous dysplasia: a case report. Imaging Sci Dent. 2011; 41:139–142.

20. Resnick CM, Novelline RA. Cemento-osseous dysplasia, a radiological mimic of periapical dental abscess. Emerg Radiol. 2008; 15:367–374.

21. Huh JK, Shin SJ. Misdiagnosis of florid cemento-osseous dysplasia leading to unnecessary root canal treatment: a case report. Restor Dent Endod. 2013; 38:160–166.

22. Eskandarloo A, Yousefi F. CBCT findings of periapical cemento-osseous dysplasia: a case report. Imaging Sci Dent. 2013; 43:215–218.

23. Said-al-Naief NA, Surwillo E. Florid osseous dysplasia of the mandible: report of a case. Compend Contin Educ Dent. 1999; 20:1017–1019.
24. Smith S, Patel K, Hoskinson AE. Periapical cemental dysplasia: a case of misdiagnosis. Br Dent J. 1998; 185:122–123.

25. Consolaro A. Florid cemento-osseous dysplasia: one of the few contraindications to osseointegrated implants. Dent Press Implantol. 2015; 9:26–33.

26. Macdonald-Jankowski DS. Focal cemento-osseous dysplasia: a systematic review. Dentomaxillofac Radiol. 2008; 37:350–360.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download