1. Maruta F, Ota H, Genta RM, Sugiyama A, Tatematsu M, Katsuyama T, Kawasaki S. Role of
N-Methyl-
N-nitrosourea in the induction of intestinal metaplasia and gastric adenocarcinoma in Mongolian gerbils infected with
Helicobacter pylori. Scand J Gastroenterol. 2001; 36(3):283–290. PMID:
11305516.
2. Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, Shimizu N, Tatematsu M.
Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998; 58(10):2067–2069. PMID:
9605743.
3. Asaka M, Sugiyama T, Kato M, Satoh K, Kuwayama H, Fukuda Y, Fujioka T, Takemoto T, Kimura K, Shimoyama T, Shimizu K, Kobayashi S. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of
Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001; 6(3):254–261. PMID:
11683930.
4. Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E. A follow-up study on the effect of
Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci. 2005; 50(8):1517–1522. PMID:
16110845.
5. Maruta F, Sugiyama A, Ishizone S, Miyagawa S, Ota H, Katsuyama T. Eradication of
Helicobacter pylori decreases mucosal alterations linked to gastric carcinogenesis in Mongolian gerbils. J Gastroenterol. 2005; 40(1):104–105. PMID:
15692797.
6. Misiewicz JJ, Harris AW, Bardhan KD, Levi S, O'Morain C, Cooper BT, Kerr GD, Dixon MF, Langworthy H, Piper D. One week triple therapy for
Helicobacter pylori: a multicentre comparative study. Gut. 1997; 41(6):735–739. PMID:
9462204.
7. Buenz EJ, Bauer BA, Schnepple DJ, Wahner-Roedler DL, Vandell AG, Howe CL. A randomized Phase I study of
Atuna racemosa: a potential new anti-MRSA natural product extract. J Ethnopharmacol. 2007; 114(3):371–376. PMID:
17889468.
8. Liu CS, Cham TM, Yang CH, Chang HW, Chen CH, Chuang LY. Antibacterial properties of Chinese herbal medicines against nosocomial antibiotic resistant strains of
Pseudomonas aeruginosa in Taiwan. Am J Chin Med. 2007; 35(6):1047–1060. PMID:
18186590.
9. Dziri S, Hassen I, Fatnassi S, Mrabet Y, Casabianca H, Hanchi B, Hosni K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J Funct Foods. 2012; 4(2):423–432.

10. Sharma G, Gohil RN, Kaul V. Cytological status of Allium hookeri Thwaites (2n=22). Genet Resour Crop Evol. 2011; 58(7):1041–1050.
11. Bae GC, Bae DY. The anti-inflammatory effects of ethanol extract of Allium hookeri cultivated in South Korea. Korean J Herbol. 2012; 27(6):55–61.
12. Kim CH, Lee MA, Kim TW, Jang JY, Kim HJ. Anti-inflammatory effect of Allium hookeri root methanol extract in LPS-induced RAW264.7 cells. J Korean Soc Food Sci Nutr. 2012; 41(11):1645–1648.
13. Won JY, Yoo YC, Kang EJ, Yang H, Kim GH, Seong BJ, Kim SI, Han SH, Lee SS, Lee KS. Chemical components, DPPH radical scavenging activity and inhibitory effects on nitric oxide production in Allium hookeri cultivated under open field and greenhouse conditions. J Korean Soc Food Sci Nutr. 2013; 42(9):1351–1356.
14. Castillo-Juárez I, Rivero-Cruz F, Celis H, Romero I. Anti-
Helicobacter pylori activity of anacardic acids from Amphipterygium adstringens. J Ethnopharmacol. 2007; 114(1):72–77. PMID:
17768020.
15. Moon DI, Shin EH, Oh HG, Oh JS, Hong S, Chung Y, Kim O. Usefulness of a
Helicobacter pylori stool antigen test for diagnosing H. pylori infected C57BL/6 mice. Lab Anim Res. 2013; 29(1):27–32. PMID:
23573105.
16. Lee HA, Hong S, Oh HG, Park SH, Kim YC, Park H, Jeong GS, Kim O. In vitro and in vivo Antibacterial Activities of Cinnamomum cassia Extracts against Helicobacter pylori. Lab Anim Res. 2010; 26(1):21–29.
17. Wroblewski LE, Peek RM Jr, Wilson KT.
Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010; 23(4):713–739. PMID:
20930071.
18. Lee EB, Kim JH, Yang JH, Kim YS, Jun HI, Ki B, Yi SH, Kim YS, Han SC, Kim DG. Antioxidant and longevity properties of the root of Allium hookeri in Caenorhabditis elegans. Kor J Pharmacogn. 2015; 46(3):234–242.
19. Li R, Wang YF, Sun Q, Hu HB. Chemical composition and antimicrobial activity of the essential oil from
Allium hookeri consumed in Xishuangbanna, southwest China. Nat Prod Commun. 2014; 9(6):863–864. PMID:
25115101.
20. Yang HS, Choi YJ, Jin HY, Lee SC, Huh CK. Effects of Allium hookeri root water extracts on inhibition of adipogenesis and GLUT-4 expression in 3T3-L1 adipocytes. Food Sci Biotechnol. 2016; 25(2):615–621.
21. Roh SS, Kwon OJ, Yang JH, Kim YS, Lee SH, Jin JS, Jeon YD, Yokozawa T, Kim HJ.
Allium hookeri root protects oxidative stress-induced inflammatory responses and β-cell damage in pancreas of streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2016; 16:63–72. PMID:
26888412.

22. Ayam VS. Allium hookeri, Thw. Enum. A lesser known terrestrial perennial herb used as food and its ethnobotanical relevance in Manipur. Afric J Food Agric Nutr Dev. 2011; 11(6):5389–5412.
23. Rhyu DY, Park SH. Characterization of Alkyl thiosulfinate in Allium hookeri root using HPLC-ESI-MS. J Korean Soc Appl Biol Chem. 2013; 56(4):457–459.
24. Kim S, Kim DB, Lee S, Park J, Shin D, Yoo M. Profiling of organosulphur compounds using HPLC-PDA and GC/MS system and antioxidant activities in hooker chive (Allium hookeri). Nat Prod Res. 2016; 30(24):2798–2804.
25. Jun HI, Jang H, Ahn D, Kim DK, Yang JH, Yun BS, Kim YS. Isolation and characterization of phenolic compound from Allium hookeri root for potential use as antioxidant in foods. Food Sci Biotechnol. 2015; 24(6):2031–2034.
26. Park H, Jeong J, Hyun H, Kim J, Kim H, Oh HI, Choi JY, Hwang HS, Oh DB, Kim JI, Kim HH. Effects of a hot-water extract of
Allium hookeri roots on bone formation in human osteoblast-like MG-63 cells
in vitro and in rats
in vivo. Planta Med. 2016; 82(16):1410–1415. PMID:
27280935.
27. Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of
Lactobacillus gasseri OLL2716 (LG21) on
Helicobacter pylori infection in humans. J Antimicrob Chemother. 2001; 47(5):709–710. PMID:
11328791.
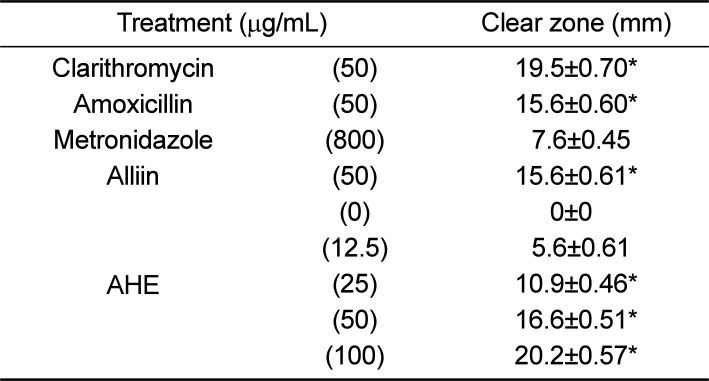
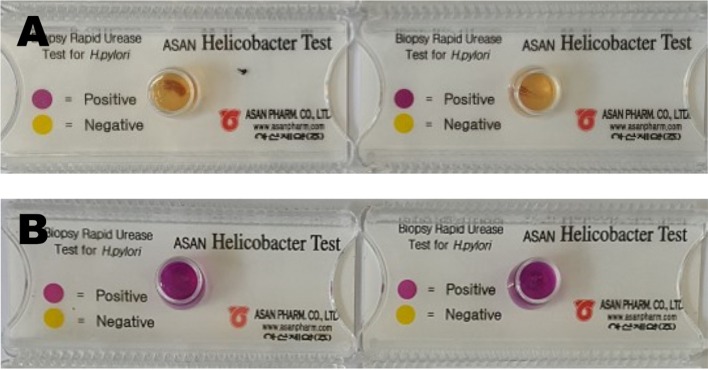
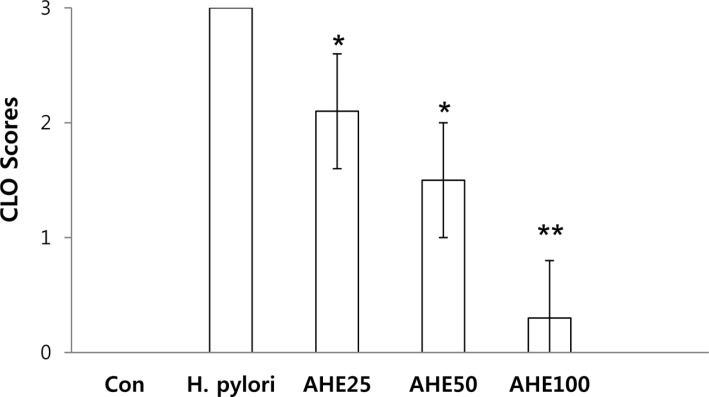
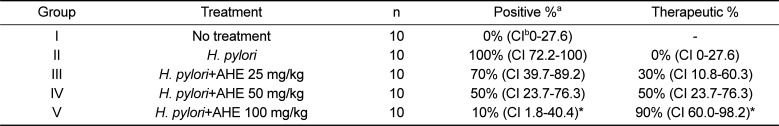




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download