Abstract
A 41-year-old man suffering from eosinophilic granulomatosis with polyangiitis (EPGA), diagnosed at another clinic on the basis of American College of Rheumatology Criteria, with a history of bronchial asthma, eosinophilia, mononeuritis multiplex, and non-fixed pulmonary infiltrates, was admitted to our department for further treatment. The patient complained of chest pain that started recently. An echocardiogram identified myocardial thickening and decreased wall motion, based on which the patient was diagnosed as having EPGA with myocarditis. The patient was successfully treated using glucocorticoids, such as methyl prednisolone (PSL) and PSL in combination with cyclophosphamide (CPM). However, CPM administration was discontinued afterwards because of the risk of bone marrow toxicity, the increased eosinophilic count (EOC) that we considered as an index of disease activity. Subsequently, the patient received additional clarithromycin (CAM) and tacrolimus (TAC) treatment considering their immunomodulatory effects. As a result, the EOC decreased and the PSL dosage could be reduced. This case shows that additional CAM and TAC treatment may be beneficial in some cases of EPGA.
Go to : 
Eosinophilic granulomatosis with polyangiitis [EGPA, Churg–Strauss syndrome (CSS)] is a multisystem disease characterized by asthma and other symptoms of allergy, necrotizing vasculitis involving small to medium-sized blood vessels and marked tissue, and peripheral blood eosinophilia. The pathogenesis of EGPA is currently unclear; however, evidence implicates eosinophils, T lymphocytes, and humoral responses as potential mediators of tissue damage.[1] Conventional treatment of EGPA is based on glucocorticoids; a combination of glucocorticoids and cyclophosphamide (CPM) is used for patients with serious organ involvement.
The cytokine interleukin (IL)-5 regulates eosinophil proliferation, maturation, and differentiation and rises to a high level in patients with EPGA. IL-5 neutralization offers a potential therapeutic option. Mepolizumab is an anti-IL-5 monoclonal antibody that binds IL-5, preventing its interaction with eosinophil surface receptors, and resulting in a consistent reduction in the absolute eosinophil count (EOC), which may lead to concomitant clinical improvement in patients with EPGA or severe eosinophilic asthma.[23]
Macrolides, such as clarithromycin (CAM) and erythromycin (EM), and calcineurin inhibitors, such as cyclosporine A (Cy-A) and tacrolimus (TAC), have been reported to exhibit immunomodulatory effects, including anti-T lymphocytic, and anti-eosinophilic effects that are thought to be related to IL-5.[45678] We herein report a case of EGPA treated with glucocorticoids in combination with CAM and TAC considering their immunomodulatory effects.
Go to : 
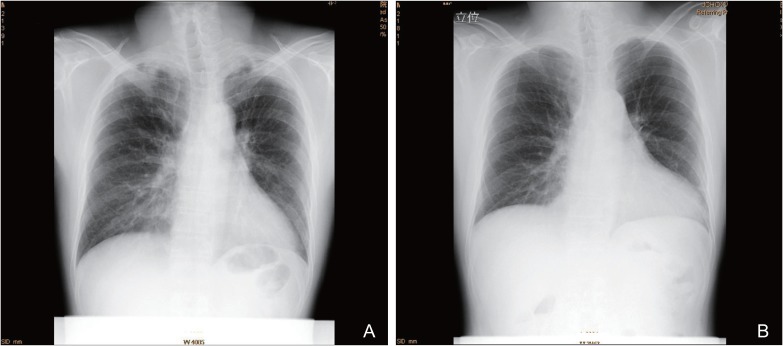
A 41-year-old man suffering from EPGA, diagnosed at another clinic on the basis of American College of Rheumatology Criteria for CSS.[9] with a history of bronchial asthma, eosinophilia, mononeuritis multiplex, and non-fixed pulmonary infiltrates on roentgenogram, was admitted to our department for further treatment. The patient complained of chest pain that started recently. On admission, the patient had slight difficulty in walking. Neurological evaluation of the patient showed muscle weakness in the bilateral quadriceps, hamstrings, and tibialis anterior muscles. He had hypesthesia in the bilateral feet. Laboratory findings were as follows: white blood cell count, 17,480/µL (eosinophils, 27.0%; EOC, 4,720/µL); hemoglobin, 13.2 g/dL; platelet count, 29.6 × 104/µL; creatine phosphokinase, 121 U/L (normal range, 50–210 U/L); and C-reactive protein (CRP), 2.42 mg/dL (normal value, <0.30 mg/dL). Neither antinuclear antibody nor rheumatoid factors were detected. Laboratory tests for myeloperoxidase and proteinase-3 antineutrophil cytoplasmic antibodies were negative. A chest roentgenogram showed infiltration shadows in bilateral upper lung fields and right lower lung field (Fig. 1A). Electrocardiography demonstrated ST-segment depression in leads II, III, aVF, V5, and V6, which suggested cardiac involvement due to EPGA.[10] At the same time, echocardiogram demonstrated myocardial thickening and decreased wall motion with left ventricular ejection fraction of 48.6% (normal value, 55%<). The patient was diagnosed as having EGPA presenting with myocarditis as well as polyneuritis multiplex. Because the patient had been treated with prednisolone (PSL) (25 mg/day) for 2 weeks at another clinic, we prescribed PSL (70 mg/day). We considered the EOC as an index of disease activity.[111] While his bronchial asthma remained stable with regular steroid inhalation before suffering from EPGA, the EOC was 200–300/µL. Therefore, we regarded the normal EOC as 200–300/µL. One week after increasing the PSL (70 mg/day) dosage, the EOC decreased to 1,140/µL; however, subsequently the EOC gradually increased to 2,660/µL. Therefore, the patient was treated with methyl PSL (1 g/day) for 3 days and subsequently with PSL (70 mg/day). At the same time, CPM (100 mg/day) was administered. Four weeks after initiating methyl PSL pulse therapy, the EOC and CRP decreased to 11/µL and 0.2 mg/dL, respectively. Although muscle weakness and hypesthesia did not improve sufficiently, chest pain, electrocardiogram, echocardiogram, and chest roentgenogram improved (Fig. 1B). Over the following 5 months, the PSL dosage was gradually decreased to 10 mg/day. Because the EOC was 34/µL on 10 mg/day of PSL, the PSL dosage was decreased to 9 mg/day. However, 8 weeks after the new PSL regimen (9 mg/day), the EOC increased to 484/µL. Therefore, the PSL dosage was increased to 15 mg/day. Two weeks after increasing the PSL (15 mg/day) dosage, the EOC decreased to 149/µL. The PSL dosage was therefore gradually decreased to 10 mg/day. During PSL (10 mg/day) treatment, the EOC remained stable (180–250/µL). Because the patient received CPM (100 mg/day) for over 1 year, CPM administration was discontinued because of the risk of bone marrow toxicity. However, two weeks after stopping CPM, the EOC increased to 358/µL. Therefore, we prescribed CAM (800 mg/day) considering its immunomodulatory effects. Six weeks after starting CAM treatment, the EOC decreased to 230/µL. Thereafter, the EOC remained stable (230–260/µL). Because of the development of slightly loose stool suggestive of an adverse reaction of CAM, its dosage was reduced to 400 mg/day. The EOC remained stable (210–270/µL). Subsequently, the administration of CAM was discontinued in order to assess its efficacy. Eight weeks after discontinuing the administration of CAM, the EOC increased to 709/µL. Therefore, the administration of CAM (400 mg/day) was resumed. The EOC gradually decreased to 260/µL over the following 5 months. The PSL dosage was reduced to 9 mg/day. Eight weeks after reducing the PSL (9 mg/day) dosage, the EOC was 240/µL. Therefore, the PSL dosage was decreased to 8 mg/day. However, eight weeks after further reducing the PSL (8 mg/day) dosage, the EOC increased to 448/µL. As an alternative to increasing the PSL dosage, we prescribed TAC (1.5 mg/day) because of its immunomodulatory effects. Since it is known that TAC blood concentrations are affected by the fat content of food, we advised the patient to take TAC 2 h before supper in order to maintain TAC levels at a fixed concentration. We considered the optimal trough level of TAC to be 5.0–10 ng/mL. The trough level of TAC (1.5 mg/day) was 4.9 ng/mL. Eight weeks after TAC treatment, the EOC decreased to 200/µL. The PSL dosage could be gradually decreased to 6 mg/day without exacerbation of the EOC. The above mentioned clinical symptoms as well as electrocardiogram, echocardiogram, and chest roentgenogram remained stable irrespective of the EOC (11–709/µL) after the patient received methyl PSL pulse therapy, followed by PSL (70 mg/day) and CPM (100 mg/day). Long-term use of CAM and TAC did cause slightly loose stool and slight headache, respectively.
Go to : 
As stated above, macrolides have immunomodulatory effects as well as anti-bacterial activity. Importantly, macrolides, such as CAM and EM, have been reported to suppress the IL-5-induced prolongation of eosinophil survival.[4] Moreover, EM and its derivatives also inhibit proliferation and induce apoptosis of T-lymphocytes that secrete IL-5.[5] Based on the abovementioned immunomodulatory effects of macrolides, we have previously reported a case of eosinophilic gastroenteritis (EGE), which relapsed on steroid reduction but improved following CAM treatment.[12]. Phaw et al. have also reported a steroid-dependent EGE female patient whose symptoms exacerbated on steroid tapering. Although there was little improvement with alternative treatments such as budesonides, azathioprine and montelukast, her symptoms significantly improved and her EOC dramatically decreased following clarithromycin treatment.[13] Amayasu et al. have conducted a trial of macrolide therapy for bronchial asthma caused by eosinophilic and neutrophilic inflammation. In this trial, patients received CAM (200 mg/day) for 8 weeks, after which symptoms, blood and sputum EOC significantly decreased. The authors concluded that CAM induced a bronchial anti-inflammatory effect associated with decreased eosinophilic infiltration.[14] Cy-A and TAC have been reported to suppress the production of IL-5 by helper T-cells.[6] Several in vitro studies have shown that TAC also inhibits IL-5-mediated survival of eosinophils.[7] TAC has been shown to inhibit NF-κB activation in peripheral T cells, leading to inactivation of T cells.[8] Niiyama et al. have reported cutaneous vasculitis due to EPGA improved with TAC treatment.[15] Watanabe et al. have reported a pediatric EPGA patient with refractory abdominal pain who was successfully treated with high dose methyl PSL pulse therapy and short-course intravenous CPM pulse therapy, followed by oral TAC and PSL.[16] Hirano et al. have reported an EPGA patient who was successfully treated with methyl PSL pulse therapy, followed by PSL (60 mg/day) and intravenous immunoglobulin (IVIG), but could not be successfully treated with subsequent PSL (22.5–55 mg/day), CPA, Cy-A, azathioprine and IVIG. Finally, the patient could be successfully treated with PSL (20 mg/day) and TAC.[17] On the basis of their case, they have concluded that TAC achieved and maintained remission, resulting in the normalization of the EOC and the reduction of PSL dosage.[17] In the present case, because additional CAM and TAC treatment reduced the EOC, anti-T lymphocytic and anti-eosinophilic effects of this regimen were thought to operate. Because additional CAM and TAC treatment was not initiated from the beginning, we could not ascertain whether or not this treatment was efficacious in induction of remission. Considering the EOC as an index of disease activity, we prescribed PSL, CAM, and TAC. However, clinical symptoms other than neuromuscular symptoms and clinical examinations remained in remission irrespective of an abnormal EOC (358–709/µL) after the patient had received methyl PSL pulse therapy, followed by PSL and CPM. Therefore, this abnormal EOC level might not be related to clinical symptoms and examinations. Because additional CAM and TAC treatment was able to reduce EOC and required PSL dosage without any sign of recurrence, this treatment was thought to be helpful in maintaining remission and minimizing the adverse effects of PSL.
Regarding the pharmacokinetic interaction between CAM and TAC, CAM is known to suppress TAC metabolism by inhibiting cytochrome P450 3A4, increasing TAC blood concentrations, and reducing expensive TAC dosage.[1819] However, repeated drug monitoring of TAC is required to prevent adverse reactions. Because only one case is reported, more research is necessary before this treatment can be adopted for a large population.
Regarding off-label use of CAM and TAC for EGPA, we obtained informed consent from the patient.
Go to : 
Notes
Conflict of interest: - Authors: No potential conflict of interest relevant to this article is reported.
- Reviewers: Nothing to declare
- Editors: Nothing to declare
Go to : 
References
1. Grayson PC, Monach PA, Pagnoux C, Cuthbertson D, Carette S, Hoffman GS, et al. Value of commonly measured laboratory tests as biomarkers of disease activity and predictors of relapse in eosinophilic granulomatosis with polyangiitis. Rheumatology (Oxford). 2015; 54:1351–1359. DOI: 10.1093/rheumatology/keu427. PMID: 25406357.

2. Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017; 376:1921–1932. DOI: 10.1056/NEJMoa1702079. PMID: 28514601.

3. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14. DOI: 10.4168/aair.2017.9.1.3. PMID: 27826957.

4. Adachi T, Motojima S, Hirata A, Fukuda T, Kihara N, Kosaku A, et al. Eosinophil apoptosis caused by theophylline, glucocorticoids, and macrolides after stimulation with IL-5. J Allergy Clin Immunol. 1996; 98:S207–S215. PMID: 8977529.

5. Wu L, Zhang W, Tian L, Bao K, Li P, Lin J. Immunomodulatory effects of erythromycin and its derivatives on human T-lymphocyte in vitro. Immunopharmacol Immunotoxicol. 2007; 29:587–596. PMID: 18075867.
6. Mori A, Kaminuma O, Ogawa K, Nakata A, Egan RW, Akiyama K, et al. Control of IL-5 production by human helper T cells as a treatment for eosinophilic inflammation: comparison of in vitro and in vivo effects between selective and non selective cytokine synthesis inhibitors. J Allergy Clin Immunol. 2000; 106:S58–S64. PMID: 10887335.
7. Hossain M, Okubo Y, Sekiguchi M. Effects of various drugs (staurosporine, herbimycin A, ketotifen, theophylline, FK506 and cyclosporine A) on eosinophil viability. Arerugi. 1994; 43:711–717. PMID: 7520689.
8. Vafadari R, Kraaijeveld R, Weimar W, Baan CC. Tacrolimus inhibits NF-κB activation in peripheral human T cells. PLoS One. 2013; 8:e60784. DOI: 10.1371/journal.pone.0060784. PMID: 23573283.

9. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome. Arthritis Rheum. 1990; 33:1094–1100. PMID: 2202307.
10. Papadimitraki ED, Moyssakis I, Mavrogeni S, Mylona M, Anagnostou D, Merkouris K, et al. A Churg-Strauss syndrome patient with myocardial involvement. J Cardiol Cases. 2015; 11:52–55. PMID: 30534258.
11. Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): clinical characteristics and long-term follow up of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013; 65:270–281. PMID: 23044708.
12. Ohe M, Hashino S. Successful treatment of eosinophilic gastroenteritis with clarithromycin. Korean J Intern Med. 2012; 27:451–454. DOI: 10.3904/kjim.2012.27.4.451. PMID: 23269887.

13. Phaw NA, Tsai HH. Eosinophilic gastroenteritis: a challenge to diagnose and treat. BMJ Case Rep. 2016; 2016:bcr2016215964. DOI: 10.1136/bcr-2016-215964.

14. Amayasu H, Yoshida S, Ebana S, YamamotoY , Nishikawa T, Shoji T, et al. Clarithromycin sup-presses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthma. Ann Allergy Asthma Immunol. 2000; 84:594–598. PMID: 10875487.

15. Niiyama S, Amoh Y, Suzuki K, Wada T, Katsuoka K. Efficacy of tacrolimus against Churg-Strauss syndrome in a patient with myasthenia gravis. Rheumatol Int. 2010; 30:847–848. DOI: 10.1007/s00296-009-1039-8. PMID: 19582460.

16. Watanabe S, Aizawa-Yashiro T, Tsuruga K, Takahashi T, Ito E, Tanaka H. Successful multidrug treatment of a pediatric patient with severe Churg-Strauss syndrome refractory to prednisolone. Tohoku J Exp Med. 2011; 225:117–121. PMID: 21914976.

17. Hirano F, Mizoguchi F, Harigai M, Miyasaka N, Kohsaka H. Tacrolimus successfully used to control refractory eosinophilic granulomatosis with polyangiitis complicated by invasive aspergillosis and chronic hepatitis B. Int J Rheum Dis. 2016; DOI: 10.1111/1756-185X.12869.

18. Ohe M, Kataoka H, Mukai M. A case of lupus nephritis treated with clarithromycin, tacrolimus, and glucocorticoids. Kaohsiung J Med Sci. 2016; 32:484–485. DOI: 10.1016/j.kjms.2016.04.007. PMID: 27638410.

19. Ohe M, Bohgaki T. A case of adult-onset Still's disease treated with monitoring of serum tacrolimus. Bull Hosp Jt Dis (2013). 2015; 73:213–216. PMID: 26535602.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download