Dear Editor:
Tumor necrosis factor (TNF)-α inhibitor has shown various adverse skin reactions, including alopecia areata, atopic dermatitis, leukocytoclastic vasculitis, and so forth. Herein, we report a rare case of segmental vitiligo (SV) during infliximab therapy and like to provide academic information about its pathogenesis.
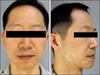
A 34-year-old man presented with multiple hypopigmented patches appeared 4 months after the initiation of intravenous infliximab therapy, as part of which he had received 4 infusions (Fig. 1). He had been treated for ulcerative colitis (UC) for 9 years showing wax and wane, but did not report any symptomatic aggravation at the time of starting infliximab therapy. Laboratory findings including antinuclear antibody (ANA) and thyroidal disease screening revealed nothing abnormal. Cutaneous lesions were distributed mainly on the right mandibular and auricular area with a patch on upper eyelid. We diagnosed as a type II SV and conducted excimer laser therapy, but he refused to pursue more treatments after two sessions.
TNF-α, a proinflammatory cytokine central to many autoimmune diseases, is increased in lesional vitiligo skin, and the tissue levels are directly correlated with the disease activity. TNF-α inhibits melanocyte proliferation and promotes melanocyte apoptosis1. These findings have provided a rationale for the use of TNF-α inhibitors in vitiligo. However, paradoxical development of de novo vitiligo with a TNF-α antagonist is reported, and the risk appears to be greater from the use of adalimumab or infliximab than from that of etanercept1.
The mechanism by which TNF-α blockers cause vitiligo is not yet fully understood. As TNF-α activates the regulatory T cells (Tregs) in vivo, TNF-α inhibitors may reduce the Tregs, which may give cytotoxic T lymphocytes the opportunity to exert an inflammatory response and may induce depigmentation. In addition, interferon (IFN)-α, which is produced by plasmacytoid dendritic cells (pDC), is increased while receiving infliximab because TNF-α inhibits the activity of pDC. IFN-α induces chemokine receptors on T cells (CXCR3) recruiting pathogenic T cells into the skin lesion2. Another explanation may lie in the development of biological auto-immunity, which is a well-known side effect of anti-TNF-α agents. The induction of cell lysis in the TNF-α-producing cells may release intracellular particles that come into contact with the immune system, producing autoantibodies such as ANAs. However, the clinical implications of the appearance of ANAs is still unclear3, as is our patient.
Upon analysis of the chronological data, the onset of vitiligo had taken place 4 months after initiation of the infliximab treatment. This suggests an association with the medication rather than mere coexistence with his UC, which have been persisted for 9 years without any skin manifestations. Snook et al.4 reported vitiligo in 1.1% of adults with UC as compared with 0.3% in the control population. However, SV is considered that the occurrence of concomitant autoimmune diseases is uncommon5. Therefore, the direct involvement of the anti-TNF-α molecule seemed very likely in our patient, but coincidence cannot be excluded.
Anti-TNF-α therapy is expected to become more popular in the future, and dermatologist should know about the risk of paradoxical development of vitiligo.
Figures and Tables
References
1. Webb KC, Tung R, Winterfield LS, Gottlieb AB, Eby JM, Henning SW, et al. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol. 2015; 173:641–650.

2. Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016; 2:e000239.

3. Ramírez-Hernández M, Marras C, Martínez-Escribano JA. Infliximab-induced vitiligo. Dermatology. 2005; 210:79–80.

4. Snook JA, de Silva HJ, Jewell DP. The association of autoimmune disorders with inflammatory bowel disease. Q J Med. 1989; 72:835–840.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download