Abstract
A 4-month-old infant presented with asymptomatic soft nodules on his right forearm, which had developed since birth. On the suspicion of nevus lipomatosus superficialis (NLS), biopsy was performed. Histopathologic findings showed monomorphic polygonal cells with abundant granular cytoplasm. Immunohistochemical stains for CD68 and vimentin were strongly positive, but were negative for S-100 protein. Based on the pathologic findings, the patient was diagnosed as non-neural granular cell tumor (NN-GCT). GCT can be divided into conventional and non-neural GCT by immunoreactivity for S-100 protein. NN-GCT is typically manifested as a well-circumscribed, papulo-nodular dermal mass, and is known to occur in a younger group than does in conventional GCT, but is rare among children. To our knowledge, there have been no case reports of NN-GCT which appeared at birth and presented as grouped nodules. Therefore, we report this interesting case of congenital NN-GCT clinically mimicking NLS.
Clinically, nevus lipomatosus superficialis (NLS) presents as groups of soft, flattened skin-colored nodules that have smooth, wrinkled, or cerebriform surfaces. Although the lesions are usually present at birth or emerge during infancy, there are reports of adult onset of NLS1. In contrast, granular cell tumors (GCTs) tend to be solitary, with multiple lesions reported in only about 10% of cases. GCTs of the skin are well circumscribed, raised, firm, and nodular2. GCTs are divided into two types; conventional GCTs that are immunoreactive for S-100 protein and non-neural GCTs (NN-GCT) that are not immunoreactive for S-100 protein3. Although clinical presentation of NN-GCT tends to occur in younger patients than does conventional GCT4, there are no reports of congenital NN-GCT. Since it was first described by LeBoit et al.5, many cases of NN-GCT have been reported; however, there have been no published cases of congenital NN-GCT clinically mimicking NLS.
A 4-month-old infant presented with asymptomatic skin lesions on the right forearm. The skin lesions had been present since birth and had grown gradually. Physical examination revealed approximately ten skin-colored, well-defined, non-tender soft papules and nodules on the distal part of his right forearm (Fig. 1). There was no sign of trauma or irritation. A skin biopsy was taken with the clinical impression of NLS. Histopathologic findings revealed diffuse infiltration of monomorphic granular tumor cells in the dermis (Fig. 2A, B). Under higher magnification, tumor cells were observed to have abundant granular cytoplasm with pleomorphic nuclei (Fig. 2C). Immunohistochemical findings showed that the biopsied skin cells were strongly positive for CD68 and vimentin, but were negative for S-100 protein and smooth muscle actin (Fig. 2D~G). The cytoplasmic granules of the tumor cells showed positive for periodic acid Schiff (PAS) staining (Fig. 2H). The patient's condition was diagnosed, on the basis of the histologic and immunohistochemical results, as congenital NN-GCT clinically mimicking NLS.
NN-GCT is clinically characterized by a painless, well-circumscribed, papulo-nodular dermal mass. In contrast, conventional GCT is usually a poorly circumscribed small nodule that is often small at presentation, but can grow up to approximately 15 mm in length. These tumors can occur at a variety of locations, such as the oral cavity and gastrointestinal tract, but most commonly occur on the skin. It is typically located on the extremities or on the head and neck.
The clinical presentation of NN-GCT occurs in a younger group than does conventional GCT; it typically develops during the fourth to sixth decades of life and is rare among children. According to a study conducted by Chaudhry and Calonje4 that analyzed 11 cases of NN-GCT, the median age of patients with this condition was 33 years, but ranged from 6 to 56 years old. Other studies have shown an age distribution of NN-GCT that ranges from 5 to 83 years6. However, there was no reported case of congenital NN-GCT.
Histologically, conventional GCT often presents as small and poorly circumscribed tumor cells located in the deep dermis. It frequently shows perineural invasion. In comparison, the tumor cells of NN-GCT are spindle shaped, ovoid, or polygonal with abundant eosinophilic granular cytoplasm. They usually have sharply circumscribed tumor cells, which can be seen in the papillary dermis4. Previous reports have described that NN-GCT shows cytologic atypia with a more diverse range of mitotic activity than does conventional GCT. However, such features do not seem to indicate risk for local recurrence or metastasis7.
The most important immunohistochemical feature that distinguishes NN-GCT from conventional GCT is the lack of S-100 protein expression. NN-GCT cells stain positive for NK1-C3, CD68, and vimentin but are not immunoreactive for other mesenchymal markers including smooth muscle antigen and desmin.
Granular cell changes have been shown to occur in many other tumors. For example, leiomyoma and leiomyosarcoma can also show granular cell features, with cigar-shaped nuclei, and tend to be immunoreactive for desmin, caldesmon, and smooth muscle antigen8. Dermatofibroma or benign fibrous histiocytoma with granular cell change have also been reported, but they tend to show overlying epidermal hyperplasia, entrapped hyaline collagen bundles and polymorphic tumor cells with irregular borders9. Melanomas with granular cell changes can show some nesting of cells or the junctional component and may be express melanocytic markers such as MART-1, MITF, or HMB-4510. However, tumors with secondary granular cell change tend to involve very limited area of the lesion and do not create true GCT6.
In conclusion, we identified congenital multiple NN-GCT that clinically mimicked NLS, on the forearm of an infant. To the best of our knowledge, this is the first published report of this condition.
Figures and Tables
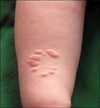 | Fig. 1Localized, grouped, skin-colored to erythematous, polygonal, soft nodules on the right forearm. |
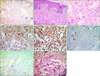 | Fig. 2(A, B) Diffuse infiltrate of monomorphic tumor cells in the dermis (H&E; A: ×100, B: ×200). (C) Large, polygonal tumor cells (arrows) with abundant granular cytoplasm (×400). (D, E) Immunohistochemical stainings revealed showing strong positivity for CD68 and vimentin (×200, respectively). (F, G) Immunohistochemical stain for S-100 and SMA were negative (S-100, ×200; SMA, ×100). (H) Periodic acid Schiff (PAS) highlights cytoplasmic granules after diastase digestion (PAS, ×400). |
References
1. Ghosh SK, Bandyopadhyay D, Jamadar NS. Nevus lipomatosus cutaneous superficialis: an unusual presentation. Dermatol Online J. 2010; 16:12.

2. Elder DE. Lever's histopathology of the skin. 11th ed. Philadelphia: Wolters Kluwer;2015. p. 1392.
3. Namkoong S, Kim JY, Gye J, Chung J, Hong SP, Kim MH, et al. Large dermal non neural granular cell tumor on the surgical wound site. Ann Dermatol. 2011; 23:Suppl 2. S147–S150.

4. Chaudhry IH, Calonje E. Dermal non-neural granular cell tumour (so-called primitive polypoid granular cell tumour): a distinctive entity further delineated in a clinicopathological study of 11 cases. Histopathology. 2005; 47:179–185.

5. LeBoit PE, Barr RJ, Burall S, Metcalf JS, Yen TS, Wick MR. Primitive polypoid granular-cell tumor and other cutaneous granular-cell neoplasms of apparent nonneural origin. Am J Surg Pathol. 1991; 15:48–58.

6. Lazar AJ, Fletcher CD. Primitive nonneural granular cell tumors of skin: clinicopathologic analysis of 13 cases. Am J Surg Pathol. 2005; 29:927–934.
7. Newton P, Schenker M, Wadehra V, Husain A. A case of metastatic non-neural granular cell tumor in a 13-year-old girl. J Cutan Pathol. 2014; 41:536–538.

8. Mentzel T, Wadden C, Fletcher CD. Granular cell change in smooth muscle tumours of skin and soft tissue. Histopathology. 1994; 24:223–231.

9. Calonje E, Fletcher CDM. Cutaneous fibrohistiocytic tumours: an update. Adv Anat Pathol. 1994; 1:2–15.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download