Abstract
Background
The human dermal papilla cells (hDPCs) play an important role in regulation of hair cycling and growth.
Objective
The aim of this study was to investigate the effect of different wavelengths of light-emitting diode (LED) irradiation on the proliferation of cultured hDPCs and on the growth of human hair follicles (HFs) in vitro.
Methods
We examined the effect of LED irradiation on Wnt/β-catenin signaling and mitogen-activated protein kinase (MAPK) pathways in hDPCs. Anagen HFs were cultured with LED irradiation and elongation of each hair shaft was measured.
Results
The most potent wavelength in promoting the hDPC proliferation is 660 nm and 830 nm promoted hDPC proliferation to a lesser extent than 660 nm. Various wavelengths significantly increased β-catenin, Axin2, Wnt3a, Wnt5a and Wnt10b mRNA expression. LED irradiation significantly increased β-catenin and cyclin D expression, and the phosphorylation of MAPK and extracellular signal-regulated kinase (ERK). HFs irradiated with 415 nm and 660 nm grew longer than control.
Conclusion
Our result suggests that LED has a potential to stimulate hDPC proliferation via the activation of Wnt/β-catenin signaling and ERK pathway. To our best knowledge, this is the first report which investigated that the effect of various wavelengths of LED on hDPC proliferation and the underlying mechanisms.
After the discovery of Endre Mester, who first observed that hair in shaven mice irradiated with a low fluence ruby laser (694 nm) grew faster than in unirradiated mice1, light-emitting diode (LED) has been clinically and experimentally reported to be useful for various medical conditions, including hair growth23. LED is an excellent alternative to deliver continuous precise wavelength of light other than laser and most studies investigating the effect of LED light on hair growth have used wavelengths that range from 635 to 650 nm. 655 nm light from Hairmax Lasercomb® (Lexington Int., LLC, Boca Raton, FL, USA) increased number of anagen hair follicles (HFs) in the C3H/HeJ mouse4, but in another study of C3H/HeJ mouse with extensive alopecia areata, the light therapy did not induce hair growth5. The effect of light on hair growth is not consistent across clinical trials and animal experiments45 and only limited ranges of wavelengths have been studied.
In this study, we demonstrated for the first time the effect of different wavelengths of LED light (415, 525, 660, and 830 nm, respectively) on the proliferation of cultured human dermal papilla cells (hDPCs) and on the growth of human HFs in vitro. Although both the Wnt/β-catenin and the mitogen-activated protein kinase (MAPK) pathways are major signaling pathways for cellular transformation, their role in the proliferation of hDPCs irradiated with LED light is rarely studied. Therefore, we assessed whether various wavelengths of LED light could regulate Wnt/β-catenin signaling and MAPK pathways in hDPCs.
A LED device was obtained from Korea Electronics Technology Institute (KETI, Seongnam, Korea). Four types of LED light sources were manufactured: 415 nm (4.23 mW/cm2); 525 nm (3.85 mW/cm2); 660 nm (2.42 mW/cm2); and 830 nm (24.72 mW/cm2) with 1, 5, or 10 J (Table 1).
hDPCs (Promocell; GmbH, Heidlberg, Germany) were cultured as previously described6. The DPCs (2×104 cells per well) were seeded into 24-well culture plates and then irradiated with LED in 10% fetal bovine serum (Gibco BRL; Life Technology, Karlsruhe, Germany), 1% penicillin/streptomycin (Gibco BRL) containing Dulbecco's modified Eagle medium (DMEM) for 48 hours. DPCs were used passage from 3 to 4 for the experiments. For LED irradiation, DPCs were seeded in on culture dish plates with serum free-DMEM and then the plates were exposed to LED light at distances of 2 cm. All dishes used in the experiments (including controls) were maintained at room temperature and atmosphere during light irradiation.
Cell proliferation was determined using a MTT assay that was performed by a slight modification of the method described by Twentyman and Luscombe7. Briefly, DPCs were seeded at a density of 2×104 cells/well into 24-well plates and irradiated with light. After 48 hours of incubation, the cells were treated with an MTT reagent for 4 hours. The samples were assessed by measuring absorbance at 540 nm with an ELISA plate reader.
Total RNA from the DPCs using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) and cDNA synthesis with QuantiTect Rev. Transcription kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The cDNA used for real time-polymerase chain reaction (RT-PCR), which was carried out with SYBR Green (Takara, Shiga, Japan). The primers sequences and PCR conditions are listed in Table 2. The PCR product was quantified with the use of analysis software (Quantity one 1-D analysis; Bio-Rad, Hercules, CA, USA).
Cells were harvested and lysed with RIPA lysis buffer (Pierce, Rockford, IL, USA). Protein concentration in the cells was measured using Bradford reagent (Bio-Rad) with bovine serum albumin (BSA) as a standard. Cell lysates containing equal amounts of total protein were separated by electrophoresis on SDS-polyacrylamide gel and then transferred to a PVDF membrane. The membranes were blocked with 5% BSA in TBS-T and subsequently incubated with primary antibodies against phospho-extracellular signal-regulated kinase (ERK), total-ERK, phospho-c-Jun N-terminal kinase (JNK), total-JNK, phosphorylated MEK, Cyclin D1 β-catenin and β-actin were obtained from Cell signaling Technology, Inc. (Cell Signal, Beverly, MA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA). Specific reactive bands were detected with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) and the immunoreactive bands were visualized by the enhanced chemiluminescence detection system (Millipore Corp., Bedford, MA, USA).
Anagen HFs from human scalp skin specimens was obtained. The medical ethical committee of the Catholic University of Korea approved specimen collection. Three HFs per well in 24-well plates were cultured in Williams E medium at 37℃ in a humidified atmosphere with 5% CO2 in 500 µl of basal medium supplemented with insulin 10 µg/ml, hydrocortisone 10 ng/ml, streptomycin 10 µg/ml, and penicillin 100 U/ml according to the Philpott's method as described8. Each experimental group contained at least 60 anagen HFs derived from one donor.
All data were expressed as mean±standard deviation (SD). Student's t-test was used to compare data between two groups, and one-way analysis of variance (ANOVA) with post hoc Duncan's multiple comparison test was used to compare data among groups. We considered a two-sided p<0.05 statistically significant. Because these were exploratory analyses, no p-value adjustment was made for multiple comparisons. All statistical analyses were performed using SigmaPlot 12.3 software.
We irradiated various wavelengths of 415 nm, 525 nm, 660 nm, and 830 nm light at doses of 1, 5 and 10 J/cm2 on hDPCs and performed MTT assay at 48 hours. All four wavelengths (415 nm of 1, 5 and 10 J/cm2, 525 nm of 1, 5 and 10 J/cm2, 660 nm of 5 and 10 J/cm2 and 830 nm of 1 and 5 J/cm2) significantly increased hDPC proliferation (Fig. 1). 660 nm at 10 J/cm2 was the most potent in promoting hDPC proliferation, as dose increased from 5 to 10 J/cm2 with a maximal effect at 10 J/cm2 (Fig. 1C).
Next, we examined changes in the mRNA expression of β-catenin, Axin2, Wnt3a, Wnt5a and Wnt10b. Both 660 nm and 830 nm significantly enhanced β-catenin mRNA expression compared to control (Fig. 2A). All four wavelengths significantly increased Axin2 expression (Fig. 2B). Wnt3a expression was increased by 660 nm and 830 nm of 10 J/cm2 (Fig. 2C) and Wnt5a expression was increased by 415nm of 1 J/cm2 and 660nm of 5 J/cm2 compared to control (Fig. 2D). Wnt10b mRNA expression was markedly enhanced as much as 10- to 15-fold by 525 nm at 10 J/cm2, 660 nm at 5 and 10 J/cm2 and 830 nm at all doses (Fig. 2E).
We also measured the protein expression of β-catenin and cyclin D1 in hDPCs. 660 nm at 5 J/cm2 induced the maximum peak expression of β- and 525 nm at 5 J/cm2 also increased β-catenin protein expression (Fig. 2F). 415 nm at 5 J/cm2, 525 nm at 10 J/cm2, 660 nm and 830 nm at all doses increased cyclin D1 protein expression (Fig. 2G).
LED light induced significant increase of mitogen-activated protein kinase kinase (MEK) and ERK phosphorylation (Fig. 3A, B), in contrast to JNK (Fig. 3C) and p38 MAPK (data not shown). MEK phosphorylation was profoundly increased as much as 5- to 6-fold by 660 nm and 830 nm at 5 J/cm2 (Fig. 3A). ERK phosphorylation was highly increased as much as 5- to 8-fold by 415 nm at 10/cm2, 525 nm at 10/cm2, 660 nm at 5 and 10 J/cm2, and 830 nm at 5 and 10 J/cm2 (Fig. 3B).
Furthermore, to determine that ERK signaling was involved in hDPC proliferation by LED light, hDPCs were pretreated with 20 µM PD98059, an ERK inhibitor, prior to light irradiation. LED light without PD98059 promoted hDPC proliferation in all wavelengths and doses (Fig. 3I~L). In the presence of the PD98059, however, the proliferative effect of LED light on hDPCs was inhibited (Fig. 3D; Statistically significant differences were determined by t-test compared to CTL (†p<0.05). 415 nm, 525 nm and 660 nm of all doses, and 830 nm of 1 and 5 J/cm2 significantly counteracted the inhibitory effect of PD98059 on hDPC proliferation (Fig. 3E~H; Statistically significant differences were determined by one-way ANOVA (#p<0.05) compared to PD98059 treatment group). Therefore, LED light promoted the hDPC proliferation by activating the ERK signaling pathway.
We performed ex vivo culture of whole human scalp HFs. Thirty nine HFs from one individual were cultured with light of various wavelengths and doses for 3 days. Elongation of each hair shaft was measured under a microscope on 3 day. The relative length of each hair shaft is shown as mean±SD from three HFs in percent change compared to control (Fig. 4). As shown in Fig. 4, HFs irradiated with 415 nm of 1 J/cm2 grew 9 % longer than control (Fig. 4A) and those with 660 nm of 1 J/cm2 and 10 J/cm2 grew 20% longer than control (Fig. 4C). HFs irradiated with 830 nm of 5 J/cm2 grew 17% longer than control (Fig. 4D). 525 nm irradiation produced no significant effect on hair shaft elongation compared to control (Fig. 4B). Statistically significant differences were determined by t-test (*p<0.05) compared to 0 day (0 day; non-treatment).
DPCs regulate development of the HF and Wnt/β-catenin pathway is considered to be essential in maintaining the hair-inducing activity of the DPC9. The peak proliferation of hDPCs by 660 nm light is consistent with recent study on mouse vibrissal DPC proliferation10. Recently, the proliferation of mouse vibrissal DPCs was known to be stimulated by red light (630 nm) irradiation10. However, other wavelengths such as 415, 525 or 810 nm also enhanced the hDPC proliferation but less potently than 660 nm light. Especially, 830 nm light promoted the hDPC proliferation to a nearly similar extent to 660 nm light. Some studies explained this result by deeper skin penetration of light with longer wavelengths11. However, considering that our experiment was performed on hDPCs in vitro unrelated to the depth of skin penetration, other studies suggesting that light-cell interaction is dependent on various cell types and wavelengths of light, might better explain our result12.
Next, we hypothesized that various wavelengths of LED light would modulate Wnt/β-catenin signaling in cultured hDPCs. β-catenin regulates hair morphogenesis and cyclin D1 regulates cell proliferation by Wnt signaling13. Only 660 nm light significantly increased the expression of β-catenin, Axin2 and all Wnt ligands in this experiment. 660 nm light also highly increased the protein level of β-catenin and cyclin D1. In recent study, Wnt10b prominently promoted the proliferation of cultured mouse DPCs in vitro, and maintained their abilities for HF induction and hair growth, while Wnt3a and Wnt5a showed limited maintenance14. In our study, 525 nm and 830 nm as well as 660 nm profoundly enhanced Wnt10b expression. LED light increased MEK and ERK phosphorylation but had no obvious effect on JNK and p38 MAPK in hDPCs. Inhibition of ERK signaling by PD98059 abrogated the proliferative effect of LED light on hDPCs. Consistent with our data, neither JNK nor p38 MAPK, but ERK alone was activated by LED light in various cell types such as mouse vibrissal DPCs and skeletal muscle cells1015. The ERK pathway transduces a cell survival pathway, but JNK is a stress-related signal pathway16. This result suggests that LED light might act as a stimulating signal which leads to hDPC proliferation via the activation of the ERK pathway, rather than a stress signal.
Our result suggests that LED light has a potential to stimulate hDPC proliferation via the activation of Wnt/β-catenin signaling and ERK pathway. To our best knowledge, this is the first report which demonstrated that the effect of various wavelengths of LED light on hDPC proliferation and the underlying mechanisms. Our study also suggests the optimal wavelengths and doses of LED light on hDPC proliferation. However, considering that HF is a complex organ composed of epithelial and mesenchymal tissues and their interaction is essential for HF growth17, more studies on outer root sheath keratinocytes and animal studies are required. In addition, further studies are necessary to investigate the possible involvement of other pathways in hDPCs induced by LED light.
Figures and Tables
 | Fig. 1Effects of various wavelengths and doses of light-emitting diode light (A: 415 nm, B: 525 nm, C: 660 nm, D: 830 nm) on human dermal papilla cell (hDPC) proliferation. The hDPCs (2×104 cells/well) were cultured in serum-free Dulbecco's modified Eagle medium for 24 hours. Then the cultured cells were irradiated immediately with indicated doses and wavelengths, and then incubated for 48 hours. MTT assay was assessed at 48 hours and the relative level is shown as mean±standard deviation from triplicate samples. Statistically significant differences were determined by one-way ANOVA (*p<0.05) compared to control (CTL). |
 | Fig. 2Effects of light-emitting diode (LED) light on the mRNA expression of β-catenin (A), Axin2 (B), Wnt3a (C), Wnt5a (D) and Wnt10b (E) in human dermal papilla cells (hDPCs). Effects of LED irradiation on the protein expression of β-catenin (F) and cyclin D1 (G) in hDPCs. The hDPCs (2×105 cells/well) were cultured in serum-free Dulbecco's modified Eagle medium for 24 hours and then irradiated with indicated doses and wavelengths, and incubated for 24 hours. The mRNA expression was examined by using real time-polymerase chain reaction and normalized against GAPDH (A~E). The protein level of β-catenin and cyclin D1 was examined by Western blot and normalized against β-actin expression (F, G). The relative level is shown as mean±standard deviation from triplicate samples. Statistically significant differences were determined by one-way ANOVA (*p<0.05) compared to control (CTL). |
 | Fig. 3Effects of light-emitting diode (LED) light on the phosphorylation of MEK (A), extracellular signal-regulated kinase (ERK) (B), and c-Jun N-terminal kinase (JNK) (C) in human dermal papilla cells (hDPCs). Effects of treatment with PD98059 on the proliferation of hDPCs and the counteract effect of LED irradiation on the inhibitory effect of PD98059 on hDPC proliferation (D~H). The hDPCs (2×105 cells/well) were cultured in serum-free Dulbecco's modified Eagle medium for 24 hours and then irradiated with indicated doses and wavelengths, and incubated for 24 hours (A~C). The protein level of phosphorylated form of MEK (MEK1/2) (A), ERK (ERK1/2) (B) and JNK (C) was examined by Western blot and the relative level is shown as mean±standard deviation (SD) from triplicate samples. Next, the hDPCs were pretreated with 20 µM of PD98059 for 1 hour, and then irradiated with indicated doses and wavelengths, and incubated for 72 hours (D~H). Positive control group in which LED irradiation without PD98059 was shown in (I~L). MTT assay was assessed at 72 hours and the relative level is shown as mean±SD from triplicate samples. Statistically significant differences were determined by one-way ANOVA (*p<0.05) compared to CTL (A~C, I~K). Statistically significant differences were determined by t-test compared to CTL (†p<0.05); one-way ANOVA (#p<0.05) compared to PD98059 treatment group (D~H). CTL: control, pMEK: phosphorylated MEK, pERK: phosphorylated ERK, pJNK: phosphorylated JNK. |
 | Fig. 4Effects of various wavelengths and doses of light-emitting diode light (A: 415 nm, B: 525 nm, C: 660 nm, D: 830 nm) on the elongation of hair follicles (HFs) in ex vivo culture of whole human scalp HFs. Thirty nine HFs from one individual were cultured with light of various wavelengths and doses for 3 days. Elongation of each hair shaft was measured under a microscope on 3 day. The relative length of each hair shaft is shown as mean±standard deviation from three HFs in percent change compared to control. Statistically significant differences were determined by t-test (*p<0.05) compared to 0 day (0 day; non-treatment). CTL: control. |
Table 1
Characteristics of light sources
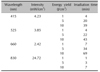
| Wavelength (nm) | Intensity (mW/cm2) | Energy yield (J/cm2) | Irradiation time (min) |
|---|---|---|---|
| 415 | 4.23 | 1 | 4 |
| 5 | 20 | ||
| 10 | 39 | ||
| 525 | 3.85 | 1 | 4 |
| 5 | 22 | ||
| 10 | 43 | ||
| 660 | 2.42 | 1 | 7 |
| 5 | 34 | ||
| 10 | 69 | ||
| 830 | 24.72 | 1 | 1 |
| 5 | 3 | ||
| 10 | 7 |
Table 2
Primers used for polymerase chain reaction amplications

ACKNOWLEDGMENT
This work was supported by the Industrial Fundamental Technology Development Program (No. 10048898) funded By the Ministry of Trade, industry & Energy (MI, Korea).
References
1. Mester E, Szende B, Gärtner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl). 1968; 9:621–626.
2. Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014; 46:144–151.

3. Lee WJ, Lee KC, Kim MJ, Jang YH, Lee SJ, Kim DW. Efficacy of red or infrared light-emitting diodes in a mouse model of propionibacterium acnes-induced inflammation. Ann Dermatol. 2016; 28:186–191.

4. Wikramanayake TC, Rodriguez R, Choudhary S, Mauro LM, Nouri K, Schachner LA, et al. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers Med Sci. 2012; 27:431–436.

5. King LE Jr, Silva KA, Kennedy VE, Sundberg JP. Lack of response to laser comb in spontaneous and graft-induced alopecia areata in C3H/HeJ mice. J Invest Dermatol. 2014; 134:264–266.

6. Messenger AG, Senior HJ, Bleehen SS. The in vitro properties of dermal papilla cell lines established from human hair follicles. Br J Dermatol. 1986; 114:425–430.

7. Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987; 56:279–285.

9. Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000; 14:1181–1185.

10. Sheen YS, Fan SM, Chan CC, Wu YF, Jee SH, Lin SJ. Visible red light enhances physiological anagen entry in vivo and has direct and indirect stimulative effects in vitro. Lasers Surg Med. 2015; 47:50–59.

11. Simpson CR, Kohl M, Essenpreis M, Cope M. Near-infrared optical properties of ex vivo human skin and subcutaneous tissues measured using the Monte Carlo inversion technique. Phys Med Biol. 1998; 43:2465–2478.

12. Weiss RA, McDaniel DH, Geronemus RG, Weiss MA. Clinical trial of a novel non-thermal LED array for reversal of photoaging: clinical, histologic, and surface profilometric results. Lasers Surg Med. 2005; 36:85–91.

13. Shimizu H, Morgan BA. Wnt signaling through the betacatenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J Invest Dermatol. 2004; 122:239–245.

14. Ouji Y, Nakamura-Uchiyama F, Yoshikawa M. Canonical Wnts, specifically Wnt-10b, show ability to maintain dermal papilla cells. Biochem Biophys Res Commun. 2013; 438:493–499.

15. Shefer G, Oron U, Irintchev A, Wernig A, Halevy O. Skeletal muscle cell activation by low-energy laser irradiation: a role for the MAPK/ERK pathway. J Cell Physiol. 2001; 187:73–80.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download