Abstract
Background
Hydroquinone (HQ) is frequently combined with retinoic acid (RA) to enhance lightening efficacy, which may also affect skin irritancy. Although skin irritation leads to postinflammatory hyperpigmentation, little research has been performed to compare skin irritancy between each component and the combination.
Objective
This study was done to examine whether HQ-RA combination increased skin irritation induced by HQ or RA alone.
Methods
Patch testing was performed using maximum therapeutic and higher concentrations of HQ and RA in 10 volunteers, and then, it was performed using their popular therapeutic concentrations and combination in the other 20 volunteers. In vitro irritation was also assessed in primary cultured normal human keratinocytes treated with 80% and 50% cell survival doses of HQ, 80% cell survival dose of RA, and their combination.
Results
The combination in patch testing induced stronger erythema than the corresponding concentrations of HQ and RA, which was remarkable with use of combination of higher concentrations. In cultured keratinocytes, the RA combination significantly decreased cell viability, but increased cytotoxicity and extracellular interleukin 1 alpha release with corresponding doses of HQ.
Hydroquinone (HQ) is frequently used in combination with retinoic acid (RA) to enhance skin lightening efficacy in hyperpigmentation skin conditions, and a triple combination of HQ, RA and steroid is popular for the treatment of hyperpigmentation skin disorders including melasma. On the other hand, HQ and RA can cause skin irritation12. Skin irritation develops frequently after use of the triple combination, although the inhibitory effect of steroid is expected. Because skin irritation could lead to postinflammatory hyperpigmentation (PIH), attention is required to assess whether the combination may increase skin irritation, particularly in pigmentary disorder-prone Latin Americans, Hispanics, and Asians with Fitzpatrick skin type III~V34. However, little research has been conducted to examine and compare skin irritation between the HQ-RA combination and each component.
Skin irritation can be induced by different mechanisms, and a multiparametric approach is recommended for the evaluation of cutaneous irritancy56. Although patch testing is a standard diagnostic method to identify the causative allergens in allergic contact dermatitis, it has also been considered as an in vivo standard method for assessing skin irritation7. There is no standardized protocol to assess irritation in vitro. However, cell viability, cytotoxicity, and extracellular release of interleukin 1 alpha (IL-1α) from cultured human skin cells, particularly keratinocytes, been considered as the endpoints for in vitro assessment of skin irritation891011.
In this study, to examine whether HQ and RA increased skin irritation when used in combination, in vivo patch testing along with in vitro assessments of cell viability, extracellular IL-1α release, and cytotoxicity were performed with various concentrations of HQ, RA, and the combination. The result of patch testing suggested that skin irritation induced by HQ and RA was increased by use of the combination, and the in vitro assessment result supported the patch testing result.
Thirty volunteers (21 males and 9 females), who have never experienced irritation and/or allergic contact dermatitis to HQ and/or RA, were included in the study. Their ages ranged from 23 to 50 years, with the average age being 28.8 years (Table 1, 2). This study was approved by the Institutional Review Board of the Dongguk University Ilsan Hospital and conducted according to the Declaration of Helsinki Ethical Principles for Medical Research after obtaining written informed consent from each volunteer (IRB no. 2013-15).
HQ is used up to a concentration of 5%1213. RA is commercially available up to a concentration of 0.1%. Initially, patch testing was performed to identify the concentration of HQ and RA that induces skin irritation. For this purpose, maximum therapeutic and higher concentrations of HQ (5% and 10% in petrolatum; Sigma-Aldrich, St. Louis, MO, USA) and RA (0.1% and 0.5% in pet, Tretinoin; Sigma-Aldrich) were applied with petrolatum control on the back of 10 volunteers using the IQ Chamber (IQ Ultra™; Chemotechnique MB Diagnostics AB, Vellinge, Sweden). The result was evaluated by visual scoring at day 2 after patch testing. Based on the report which suggested that uniformity of erythema across the test site has been found to be more closely linked to the actual intensity of response14 and that skin irritation reactions including erythema have been rated on a simple scale15, patch test reactions in this study were rated on a scale as +/− (mild erythema without uniformity), +1 (mild erythema with uniformity), +2 (moderate erythema with uniformity), and +3 (severe erythema with uniformity with/without edema).
Next time, after referring to the first patch test results, patch testing was performed using therapeutic concentrations of HQ (2%, 4%, and 5% pet), RA (0.01%, 0.025%, and 0.05% pet), and their combination (HQ 2%-RA 0.01%, HQ 2%-RA 0.025%, HQ 2%-RA 0.05%, HQ 4%-RA 0.01%, HQ 4%-RA 0.025%, HQ 4%-RA 0.05%, HQ 5%-RA 0.01%, HQ 5%-RA 0.025%, and HQ 5%-RA 0.05% pet) in the other 20 volunteers and evaluated by using the same visual scoring scale.
Monolayer keratinocyte cultures were done using adult human skin specimens obtained from Caesarean-section scars and circumcision. Individual epidermal cells were suspended in EpiLife Medium (Thermo Fisher Scientific, Rockford, IL, USA) supplemented with bovine pituitary extract, bovine insulin, hydrocortisone, human epidermal growth factor, and bovine transferrin (HKGS; Thermo Fisher Scientific). The keratinocytes were seeded at 1.5×105 cells/well in 6-well plates for 1 day and were treated with various concentrations of HQ (Sigma-Aldrich), RA (Sigma-Aldrich) or HQ-RA combination dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich) for 2~3 days.
The cultured cells were stained with 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) for 4 hours in order to assess cell viability. The precipitated formazan was dissolved in DMSO (Sigma-Aldrich) and optical density was measured using a spectrophotometer at 570 nm, with background subtraction at 630 nm. Cell viability was calculated as the ratio of cell growth induced by RA, HQ or their combination to that induced by solvent. The culture supernatants were harvested and were assayed by tests for cytotoxicity using the lactate dehydrogenase (LDH; Roche, Penzberg, Germany) release method and for extracellular levels of IL-1α release using ELISA kit (R&D Systems, Minneapolis, MN, USA). All experiments were repeated at least three times.
In 10 volunteers who were patch tested with HQ (5% and 10% pet) and RA (0.1% and 0.5% pet), RA did not induce uniform erythema across the patch test sites (scale ≥+1) at 0.1% and 0.5% concentrations in 8 and 7 of the 10 volunteers, respectively, whereas HQ induced uniform erythema across the patch test sites in 6 and 8 volunteers at 5% and 10% concentrations, respectively. No reaction developed in 4 volunteers in patch testing with RA 0.1% and 0.5% and in 2 volunteers in testing with HQ 5% and 10% (Table 1, Fig. 1).
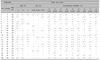
From a practical viewpoint, it was necessary to examine skin irritancy caused by the therapeutic concentrations of HQ (2%, 4%, and 5%) and/or RA (0.01%, 0.025%, and 0.05%), and these concentrations were chosen for patch testing, which was performed in the other 20 volunteers. As expected from the result of no erythematous reactions in half of the volunteers patch tested with RA even at 0.5% concentration (Table 1, Fig. 1), which suggested that there was less possibility of irritation in patch testing with popular therapeutic concentrations of RA, RA 0.05% as well as 0.025% and 0.01% did not induce erythematous patch test reactions in 14, 16, and 14 of the 20 volunteers, respectively. The erythematous reaction showed uniformity only in 1 to 2 volunteers (Table 2). On the other hand, no significant difference was observed between patch test reactions with use of 5% concentration and patch test reactions with use of 4% concentration in case of HQ (Table 2, Fig. 2). The combination induced higher erythematous reaction scores than summing up of each score with use of HQ 5%, 4% and 2% and RA 0.05% in 13, 15, and 11 of the 20 volunteers, respectively. On the other hand, an increase in the patch test reaction scores with use of the combination was observed in 3 of the 20 volunteers tested with HQ 2% and RA 0.01% combination (Table 2, Fig. 2).
For in vitro irritation assessment, primary cultured normal human keratinocytes were treated with 80% and 50% cell survival doses of HQ and 80% cell survival dose of RA, which were determined by MTT assay at 48 hours post-treatment, and with their combination, and then tested for MTT assay, LDH release, and IL-1α release 48 hours and 72 hours after the treatment. MTT assay showed that the combination of either dose of HQ with RA compared to each component significantly decreased the number of viable keratinocytes at 48 hours and 72 hours post-treatment (p<0.05, Fig. 3A). In LDH release cytotoxicity assay, the combination of 50% cell survival dose of HQ with RA increased the level (p<0.05), although the combination of 80% cell survival dose of HQ with RA did not increase the level (Fig. 3B). In addition, the combination of either dose of HQ and RA increased extracellular release of IL-1α (p<0.05, Fig. 3C).
This study examined whether therapeutic concentrations of HQ increased skin irritation when used in combination with therapeutic concentrations of RA. For the diagnosis of allergic contact dermatitis induced by HQ and RA, which is rare1216, patch test concentration and vehicle have been recommended as 1% pet and 0.005% alc, respectively17. Although accurate reliable methods for patch testing to assess skin irritation have not been established, different concentrations from those that cause allergic contact dermatitis were considered necessary for testing. In this study, therapeutic concentrations of HQ, RA, and the combination were used, considering that the skin-lightening agents containing the HQ and RA combination are supposed to be used for a long duration to treat or control hyperpigmented lesions, particularly on the face. A repeated open application test (ROAT) could be more reasonable than patch testing to identify irritation, and there are a couple of reports that compared ROAT with patch testing with respect to skin irritation. Regarding the occlusion duration of patch testing, 4-hour patch test has been proposed to evaluate the skin irritation potential18. However, the report which identified the correlation between ROAT and patch testing using topical drugs suggests that there is no difference between occlusion for 24 hours and occlusion for 48 hours19. In the report showing no correlation between ROAT and patch testing, different occlusion durations of patch testing, such as 4 hours, 24 hours, and 48 hours, had no impact on the result20. Therefore, it was reasonable to apply 48-hour occlusion for patch testing in this study.
HQ was used at a concentration of 2% up to 5%1213. RA is commercially available from 0.01% to 0.1%, and it has been used at an approximate concentration of 0.05% for management of skin hyperpigmentation. The patch test result using maximum therapeutic and higher concentrations of HQ showed that erythema of various intensities was induced with 5% concentration without a remarkable difference between 5% and 10% concentrations (Table 1, Fig. 1). No difference in the reaction was observed in patch testing using 5% and 4% concentrations of HQ (Table 2). These results suggested that therapeutic concentrations of HQ could induce irritant patch test reactions. As expected from the result of the first patch testing using maximum therapeutic and higher concentrations of RA (Table 1, Fig. 1), 0.05% or lower concentrations of RA induced skin irritation in a small number of volunteers (Table 2). Enhanced erythematous reaction induced by the HQ-RA combination compared to the corresponding concentrations of HQ could be easily detected, if RA was combined at concentrations that induced no irritant reactions. Even if RA and HQ induced erythematous patch test reactions, the reaction intensities could be judged by the score of patch test reactions. Considering that higher scores indicated more skin irritation, the combination induced higher scores than summing up of scores with use of HQ and RA in a higher number of volunteers, when higher concentrations of HQ and RA were used in combination (Table 2, Fig. 2). The result suggested that the combination of higher concentrations of HQ and RA, as in commercialized topical agents with triple combination which contain 4% or higher concentrations of HQ and 0.03% or higher of RA, could induce more skin irritation. To minimize skin irritation induced by the HQ-RA combination, particularly in pigmentary disorder-prone individuals, it might be better to use 4% or higher concentrations of HQ alone. For combination, reducing the concentrations of each ingredient might be another way to reduce skin irritation.
MTT assay, LDH assay, and IL-1α release have been considered as reliable in vitro tests for assessing skin irritation 891011. Although the exact percentage of cell survival or cytotoxicity, which is indicative of the irritation in vivo, has not been defined, doses that inhibit cell viability by 50% have been used as the threshold dose that causes irritation of human skin21. Therefore, choosing a 80% cell survival dose of RA, which is supposed to be a non-irritating dose, may be reasonable to support the in vivo patch testing result. The irritant reactions lasted for more than 4 days after patch removal following use of HQ and the combination, whereas the reactions regardless of the severity did not last for 4 days following use of RA (data not shown), suggesting the role of different irritation mechanisms between HQ and RA. Although the in vitro results suggested that the combination of a non-irritating dose of RA significantly decreased cell survival with either a non-irritating or a irritating dose of HQ, whereas it significantly increased cytotoxicity and extracellular IL-1α release induced by either dose of HQ (Fig. 3) may not provide any clue for the mechanism, the in vitro results supported the claim that the combination could increase skin irritation.
Collectively, the in vivo result of patch testing and the in vitro result of cell viability, cytotoxicity, and IL-1α release suggested that HQ and RA increased skin irritation when used in combination.
Figures and Tables
Fig. 1
Results of patch testing with maximum therapeutic and higher concentrations of hydroquinone (HQ), retinoic acid (RA), and their combination. Representative patch test reactions induced by different concentrations of HQ and RA at day 2 in three of the 10 volunteers. 1: RA 0.1% pet, 2: RA 0.5% pet, 3: HQ 5% pet, 4: HQ 10% pet, F: female, M: male.
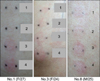
Fig. 2
Results of patch testing with popular therapeutic concentrations of hydroquinone (HQ), retinoic acid (RA), and their combination. (A) Representative patch test reactions induced by different concentrations of HQ, RA, and HQ-RA combination at day 2 in two of the 20 volunteers. Each number points to its horizontal and vertical coordinates (1: HQ 5% pet, 2: HQ 4% pet, 3: HQ 2% pet, 4: RA 0.05% pet, 5: RA 0.025% pet, 6: RA 0.01% pet, 7: HQ 5%-RA 0.05% pet, 8: HQ 5%-RA 0.025% pet, 9: HQ 5%-RA 0.01% pet, 10: HQ 4%-RA 0.05% pet, 11: HQ 4%-RA 0.025% pet, 12: HQ 4%-RA 0.01% pet, 13: HQ 2%-RA 0.05% pet, 14: HQ 2%-RA 0.025% pet, 15: HQ 2%-RA 0.01% pet). (B) The number of volunteers who increased the patch test reaction scores by the combination of corresponding concentrations. M: male.

Fig. 3
Effect of retinoic acid (RA) combination on cell viability, cytotoxicity, and extracellular interleukin 1 alpha (IL-1α) release induced by hydroquinone (HQ) in primary cultured human keratinocytes. (A) MTT assay in cultured keratinocytes treated with two different doses (80% and 50% survival doses determined by MTT assay at 48 hours) of HQ, a fixed dose (50% survival dose) of RA, and their combination for 48 hours and 72 hours (B) lactate dehydrogenase (LDH) release and (C) ELISA for IL-1α release in the culture supernatants. Data in the graph represent mean±standard deviation of relative values compared to solvent-treated control for starting point or absolute values from 4 independent experiments. *p<0.05 vs. solvent-treated control, #p<0.05 vs. HQ-treated cells for the corresponding dose, §p<0.05 vs. RA-treated cell.

Table 1
Results of patch testing at day 2 with maximum therapeutic and higher concentrations of HQ, RA, and their combination in 10 volunteers
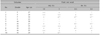
Table 2
Results of patch testing in the other 20 volunteers with therapeutic concentrations of HQ, RA, and their combination
ACKNOWLEDGMENT
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HN15C0102).
References
1. Matsubayashi T, Sakaeda T, Kita T, Kurimoto Y, Nakamura T, Nishiguchi K, et al. Intradermal concentration of hydroquinone after application of hydroquinone ointments is higher than its cytotoxic concentration. Biol Pharm Bull. 2003; 26:1365–1367.

2. Stratigos AJ, Katsambas AD. The role of topical retinoids in the treatment of photoaging. Drugs. 2005; 65:1061–1072.

3. Hexsel D, Arellano I, Rendon M. Ethnic considerations in the treatment of Hispanic and Latin-American patients with hyperpigmentation. Br J Dermatol. 2006; 156:Suppl 1. 7–12.

4. Ho SG, Chan HH. The Asian dermatologic patient: review of common pigmentary disorders and cutaneous diseases. Am J Clin Dermatol. 2009; 10:153–168.
5. van Ruissen F, Le M, Carroll JM, van der Valk PG, Schalkwijk J. Differential effects of detergents on keratinocyte gene expression. J Invest Dermatol. 1998; 110:358–363.

6. Fluhr JW, Darlenski R, Angelova-Fischer I, Tsankov N, Basketter D. Skin irritation and sensitization: mechanisms and new approaches for risk assessment. 1. Skin irritation. Skin Pharmacol Physiol. 2008; 21:124–135.

8. Osborne R, Perkins MA. In vitro skin irritation testing with human skin cell cultures. Toxicol In Vitro. 1991; 5:563–567.

9. Korting HC, Schindler S, Hartinger A, Kerscher M, Angerpointner T, Maibach HI. MTT-assay and neutral red release (NRR)-assay: relative role in the prediction of the irritancy potential of surfactants. Life Sci. 1994; 55:533–540.

10. Corsini E, Galli CL. Cytokines and irritant contact dermatitis. Toxicol Lett. 1998; 102-103:277–282.

11. Wilhelm KP, Böttjer B, Siegers CP. Quantitative assessment of primary skin irritants in vitro in a cytotoxicity model: comparison with in vivo human irritation tests. Br J Dermatol. 2001; 145:709–715.

12. Engasser PG, Maibach HI. Cosmetic and dermatology: bleaching creams. J Am Acad Dermatol. 1981; 5:143–147.
13. Yoshimura K, Momosawa A, Aiba E, Sato K, Matsumoto D, Mitoma Y, et al. Clinical trial of bleaching treatment with 10% all-trans retinol gel. Dermatol Surg. 2003; 29:155–160. discussion 160.

14. Nicholson M, Willis CM. The influence of patch test size and design on the distribution of erythema induced by sodium lauryl sulfate. Contact Dermatitis. 1999; 41:264–267.

15. Basketter D, Reynolds F, Rowson M, Talbot C, Whittle E. Visual assessment of human skin irritation: a sensitive and reproducible tool. Contact Dermatitis. 1997; 37:218–220.

16. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003; 4:473–492.
17. De Groot AC, Frosch PJ. Patch test concentrations and vehicles for testing contact allergens. In : Frosch PJ, Menné T, Lepoittevin JP, editors. Contact dermatitis. 4th ed. Berlin/Heidelberg: Springer-Verlag Berlin Heidelberg;2006. p. 907–928.
18. York M, Griffiths HA, Whittle E, Basketter DA. Evaluation of a human patch test for the identification and classification of skin irritation potential. Contact Dermatitis. 1996; 34:204–212.

19. Horita K, Tanoue C, Yasoshima M, Ohtani T, Matsunaga K. Study of the usefulness of patch testing and use test to predict the safety of commercial topical drugs. J Dermatol. 2014; 41:505–513.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download