Abstract
Background
Acne vulgaris is a disease of the pilosebaceous unit characterized by increased sebum production, hyperkeratinization, and immune responses to Propionibacterium acnes (PA). Here, we explore a possible mechanism by which a lipid receptor, G2A, regulates immune responses to a commensal bacterium.
Objective
To elucidate the inflammatory properties of G2A in monocytes in response to PA stimulation. Furthermore, our study sought to investigate pathways by which lipids modulate immune responses in response to PA.
Methods
Our studies focused on monocytes collected from human peripheral blood mononuclear cells, the monocytic cell line THP-1, and a lab strain of PA. Our studies involved the use of enzyme-linked immunosorbent, Western blot, reverse transcription polymerase chain reaction, small interfering RNA (siRNA), and microarray analysis of human acne lesions in the measurements of inflammatory markers.
Results
G2A gene expression is higher in acne lesions compared to normal skin and is inducible by the acne therapeutic, 13-cis-retinoic acid. In vitro, PA induces both the Toll-like receptor 2-dependent expression of G2A as well as the production of the G2A ligand, 9-hydroxyoctadecadienoic acid, from human monocytes. G2A gene knockdown through siRNA enhances PA stimulation of interleukin (IL)-6, IL-8, and IL-1β possibly through increased activation of the ERK1/2 MAP kinase and nuclear factor kappa B p65 pathways.
Acne vulgaris is a common skin disease that affects upwards of 85% of adolescents and frequently persists into adulthood. It is a disease of the pilosebaceous unit that is classically associated with hyperkeratinization, increased sebum production, and an inflammatory cytokine response thought to be triggered by the commensal bacterium Propionibacterium acnes (PA)12. Its pathogenesis is multifactorial and thought to involve complex and poorly understood lipid-associated pathways. Some observational studies show that abnormal sebum lipid composition is associated with worsened disease and that acne patients have low linoleate levels in skin while high levels prevent comedogenesis3. PA also metabolizes triglycerides into free fatty acids (FFAs) in the pilosebaceous unit and the density of PA is positively correlated with sebum secretion rate4. However, how lipids are directly involved in inflammatory responses seen in acne remains unclear.
G2A, also known as G protein-coupled receptor 132 (GPCR 132), is a stress-inducible GPCR and known receptor for oxidized lipids, with especially high affinity for the linoleic acid derivative 9-hydroxyoctadecadienoic acid (9-HODE). Linoleic acid is found in relatively high concentrations in sebum and is one of the FFAs known to undergo beta-oxidation in the pilosebaceous unit. In part due to its recent discovery, the immunologic roles of G2A still remain unclear. Models of atherosclerosis suggest an anti-inflammatory role as mice lacking G2A had greater accumulation of macrophages on the aortic wall, increased nuclear localization of nuclear factor-κB (NF-κB) p65 subunit, and increased IL-6 and monocyte chemoattractant protein-15. In contrast, studies in keratinocytes suggest a more pro-inflammatory role6.
Its role in skin disease and in response to microflora are emerging areas of interest. G2A was recently shown to mediate interactions between the host and gastrointestinal microflora. Bacteroides induced endogenous stimulation of G2A and is thought to quell immune responses; thus, G2A may also have a role in autoimmunity7. As G2A is also expressed in skin, it is hypothesized that this GPCR may also play a role in regulating the skin's immune response to environmental factors and the interaction between skin and its microflora128.
Our study is the first to investigate the role of G2A in the context of a commensal skin bacterium. Studying G2A in the context of PA provides an opportunity for the understanding of acne pathogenesis and G2A as an immune regulator. First, while PA colonization, sebum production, and inflammatory cytokine induction are inextricably linked in acne91011, the consequences of lipid involvement and inflammation potentially cross-regulating pathways at a sight of colonization remains elusive. Second, understanding the role of G2A in response to PA will help define the immunomodulatory properties of this receptor in the context of bacteria and help delineate underlying mechanisms by which inflammatory responses to a commensal organism can be mediated12. Our study uses human monocytes to characterize these properties of G2A and also considers the potential clinical relevance of G2A in acne.
Human monocyte isolation followed protocol described by Schenk et al.13. Peripheral blood mononuclear cells (PBMCs) obtained from donors after consent approved by the UCLA Institutional Review Board in accordance with Declaration of Helsinki Principles. PBMCs isolated using Ficoll (GE Healthcare, Uppsala, Sweden) gradient centrifugation. Monocytes enriched with Percoll density gradient (GE Healthcare) and adherence in RPMI 1640 medium (Thermo Fisher Scientific) with 1% fetal calf serum (FCS; Omega Scientific, Tarzana, CA, USA) for 2 h. Adherent monocytes cultured in 10% FCS. Monocyte purity >80% as measured by CD14 expression.
PA strain 6919 obtained from American Type Culture Collections (ATCC; Manassas, VA, USA) and grown on brucella agar with 5% sheep blood, hemin, and vitamin K (Thermo Fisher Scientific Remel Productions, Lenexa, KS, USA) at 37℃ for 3~5 days in sealed chambers with Anaero Packs (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan). Cultures grown under same conditions in reinforced clostridial medium (Oxoid, Basingstoke, England). Spectrophotometer OD600 used to determine bacterial log phase. Cultures diluted to final concentration of 0.5 multiplicity of infection (MOI) to stimulate cells.
Supernatants from monocytes stimulated for 24 hours with 0.5 MOI PA were processed to measure concentrations of HODEs and hydroxyeicosatetraenoic acids (HETEs) using method adopted from Imaizumi et al.14 LC/MS/MS performed using 4000 QTRAP (Applied Biosystems, Foster City, CA, USA) with electrospray ionization (ESI) source. High-performance liquid chromatography (HPLC) system used Agilent 1200 series LC pump with thermostatted autosampler (Agilent Technologies, Santa Clara, CA, USA). Chromatography performed using Luna C-18(2) column (3 µm particle, 150×3.0 mm). Data acquisitions and instrument control done with Analyst 1.4.2 software (Applied Biosystems). Detection accomplished with multiple reaction monitoring modes with negative ion detection. Collision energy, declustering potential, and collision cell exit potential optimized for each compound to obtain optimum sensitivity. Transitions monitored were mass-to-charge ratio (m/z): m/z 295.1→194.8 for 13-HODE; 295.0→171.0 for 9-HODE; 319.1→219.0 for 15-HETE; 319.1→115.0 for 5-HETE; 319→179 for 12-HETE.
IL-6, IL-8, and IL-1β levels in culture supernatants quantified by enzyme-linked immunosorbent assay (ELISA) using cytokine-specific capture antibodies and secondary biotin-conjugated antibodies (Thermo Fisher Scientific) in reference to recombinant proteins (eBioscience, San Diego, CA, USA) in 96-well plates (Corning, NY, USA). Plates blocked using phosphate-buffered saline (PBS) solution with 10% bovine serum albumin (BSA; Thermo Fisher Scientific, Waltham, MA, USA). Plates washed with PBS containing 0.005% Tween 20 (Sigma-Aldrich, St. Louis, MO, USA). Samples were assayed in triplicates.
Total RNA isolated using Trizol reagent (Thermo Fisher Scientific) following manufacturer's recommendations and treated with RNase-free DNase. RNA isolated with Quick-RNA MiniPrep kit (Zymo Research, Irvine, CA, USA). RNA concentration and quality determined by spectrophotometry at OD460 and OD480. Samples reverse transcribed with iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) at 25℃ 5 minutes, 42℃ 30 minutes, and 85℃ 5 minutes. Quantitative polymerase chain reaction (qPCR) performed using iQ SYBR Green Supermix (Bio-Rad) (40 cycles of 95℃ 5 minutes, 95℃ 10 seconds, 55℃ 20 seconds, and 72℃ 20 seconds). Glyceraldehyde-3-phosphate dehydrogenase amplification used as internal standard. Primers designed using Primer Express (Applied Biosystems). Gene expression level quantified by comparative method 2−ΔΔCT.
Cells for detection of G2A treated with 0.5 MOI PA, isotretinoin, and/or all-trans-retinoic acid (ATRA) incubated with anti-G2A polyclonal antibody (MBL International; Woburn, MA, USA), Immunoglobulin (Ig)G isotype control (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and secondary Alexa Fluor 488-conjugated antibody (Thermo Fisher Scientific). Between incubation steps, cells washed with PBS containing 1% bovine serum albumin. Cells fixed with 2% paraformaldehyde (Thermo Fisher Scientific), acquired on BD FACScan System, and analyzed using CellQuest-Pro software (BD, Franklin Lakes, NJ, USA).
Small interfering RNA (siRNA) transfection performed with Lonza Nucleofector system with either the Lonza Human Monocyte Nucleofector kit or Lonza Cell Line Nucleofector kit V for human promyelocytic cell line THP-1 (Lonza, Basel, Switzerland). Scramble control and anti-G2A siRNA constructs were used at 1 µg per transfection (Thermo Fisher Scientific). Transfection efficiency of expression constructs assessed using the pmax-GFP construct (Lonza), and yielded average of 50% transfection rate.
Detections of phospho-p44/42 mitogen-activated protein kinase (MAPK), phospho-p38 MAPK, and NF-κB p65 were performed using western blot. After PA treatment, cells lysed in buffer containing 1% Triton X-100 (Sigma-Aldrich) and Protease/Phosphotase Inhibitor Cocktail (Cell Signaling, Danvers, MA, USA). Protein concentrations assessed with Quick Start Bradford Protein Assay kit following manufacturer's protocol. Lysates loaded with Laemmli Sample Buffer onto Any kD Mini-PROTEAN-TGX pre-cast polyacrylamide gel (Bio-Rad) and separated by electrophoresis. Gels transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA) and detected with primary antibodies for MAPK (Cell Signaling) or NF-κB (Sigma-Aldrich) pathways, secondary horse radish peroxidase-conjugated antibody (Abcam, Cambridge, England), and chemiluminescent substrate (Thermo Fisher Scientific) before visualization with ChemiDoc MP System (Bio-Rad).
Total RNA from five acne lesions and five matched non-lesional controls was obtained from Diane Thiboutot (Pennsylvania State University, Hershey, PA, USA)15. Biopsies taken from backs of men and women without other skin diseases in biopsy areas. Gene expression profiles were re-measured using the Affymetrix GeneChip, Human Genome (HG)-U133A Plus 2.0 array platform to include GPR132, which was not included in the Trivedi et al. report15. Data analyzed as previously described16. Analysis identified 890 genes that were significantly elevated in acne lesions (fold-change≥1.5 and p<0.05), compared to controls.
Neutralizing antibodies against Toll-like receptor 2 (TLR2) and TLR4, and control antibodies (BioLegend, San Diego, CA, USA); 9(S)-HODE (Cayman Chemical Company, Ann Arbor, MI, USA); human acute monocytic leukemia cell line THP-1 (ATCC) cultured in RPMI Medium 1640 supplemented with 10% FCS, 1% Sodium Pyruvate (Thermo Fisher Scientific), and Penicillin-Streptomycin-L-Glutamine (Corning); Tris/Glycine and Tris/Glyine/SDS buffers and blotting-grade blocker for western blot (Bio-Rad); all-trans-retinoic acid (retinoic acid) and isotretinoin (13-cis-retinoic acid [13cRA]) (Sigma-Aldrich) reconstituted in dimethyl sulfoxide (Thermo Fisher Scientific).
Results expressed as means±standard error (SE) for the number of separate experiments indicated in each Fig. 1, 2, 3, 4, 5. Post hoc two-tailed Student's t-test used for comparison between two groups. For Fig. 4, 5B, to address increased likelihood of Type I errors when making comparisons over multiple groups, p-values adjusted using Bonferroni correction. For Fig. 5A, post hoc two-tailed paired t-test performed to ensure analysis of paired samples. Significant differences considered for those with p-values ≤0.05.
To test G2A expression under different conditions, we examined the effects of 9(S)-HODE and PA on THP-1 cells and primary human monocytes. G2A mRNA expression or the population of cells expressing G2A in either THP-1 or monocytes was unchanged after treatment with different concentrations of 9(S)-HODE (Fig. 1A). In contrast, PA exposure increased the population of THP-1 cells expressing G2A approximately 35-fold compared to isotype control (Fig. 1B), and increased the gene expression of GPR132 in both THP-1 and monocytes by approximately 50% and 40%, respectively (Fig. 1A, p<0.05). We next assessed the effect of TLR2, an important mediator of innate immune system in response to PA17. We observed a significant 30% reduction in GPR132 expression in monocytes pre-treated with TLR2 neutralizing antibody prior to addition of PA, as compared to the isotype control (Fig. 1C, p<0.01).
9-HODE is a known ligand of G2A12. We sought to characterize the baseline levels of this ligand and related oxidized lipids produced from human monocytes as well as their inducibility with PA. Monocytes were treated with PA for 24 hours; then cell lysates were purified and analyzed with liquid chromatography and tandem mass spectrometry. We measured the oxidized forms of linoleic acid, 9-HODE and 13-HODE, as well as three oxidized forms of another FFA, arachidonic acid, 5-, 12-, and 15-HETE. Both 9-HODE and 13-HODE were found in significantly higher concentrations (5-fold and 10-fold, respectively) than all three HETEs. Exposing monocytes to PA increased the concentration of 9-HODE by approximately 2-fold (Fig. 2, p<0.001), whereas 13-HODE was not significantly altered. Of the three HETEs measured, only 15-HETE was found to be significantly upregulated by PA (p<0.05); however, its concentration was notably less than the concentrations of both the HODEs.
To understand the immunomodulatory functions of G2A, we transfected THP-1 cells using siRNA oligonucleotides specific to G2A mRNA (siG2A), then measured gene expression and protein secretion of inflammatory cytokines IL-6, IL-8, and IL-1β, following exposure to PA. Our transfection methods consistently yielded approximately 50% knockdown of G2A mRNA expression when compared to the non-targeting oligonucleotide control (siCTRL) (Supplementary Fig. 1, p<0.05). Knockdown of the G2A gene in THP-1 cells led to an increase in inflammatory cytokines both in response to PA and G2A ligand 9(S)-HODE. A 24 hours incubation of siG2A transfected THP-1 cells with live PA resulted in increases of approximately 1.8-fold for IL-6 (Fig. 3A, p<0.01) and 1.3-fold for IL-8 (Fig. 3C, p<0.05), but no change in IL-1β; when compared to control (Fig. 3E). At the mRNA level, G2A knockdown in THP-1 cells stimulated with PA resulted in approximately 2.2-fold increase of IL6 (Fig. 3B, p<0.01), 3.9-fold increase of IL8 (Fig. 3D, p<0.01), and 1.9-fold increase of IL1B (Fig. 3F, p<0.05) mRNA when compared to control. G2A knockdown THP-1 cells treated with 9(S)-HODE exhibited increases of approximately 2.7-fold for IL6 (Fig. 3B, p<0.05), 1.2-fold increase for IL8 (Fig. 3D, p<0.05), and 1.4-fold increase for IL1B (Fig. 3F, p<0.001) when compared to control. At the protein level, there was nearly a 2.3-fold increase of IL-8 (Fig. 3C, p<0.05) when comparing PA-stimulated cells transfected with siG2A to siCTRL, while levels of IL-6 and IL-1β were not significantly changed.
The MAPK and NF-κB pathways are thought to be integral in the regulation of inflammatory cytokines IL-6 and IL-8. To elucidate the signaling pathways that are activated by G2A to regulate inflammatory response, we measured the phosphorylation of two MAPK pathways, ERK1/2 (p44/42) and p38 MAPK, and NF-κB p65 using western blot. Densitometric analysis of blot bands showed that the G2A knockdown in monocytes stimulated with PA exhibited approximately 2.3-fold increase in the phosphorylation of the ERK1/2 MAPK pathway compared to control (Fig. 4A, p<0.01), and nearly a 1.5-fold increase in NF-κB p65 subunit (Fig. 4C, p<0.05). PA induced approximately a 50% increase in the phosphorylation of p38 MAPK in the scramble control group (Fig. 4B, p<0.05); however, this was not found to be significantly modulated by the G2A knockdown.
To explore the potential clinical implications of G2A in acne, we compared the mRNA profile of acne lesions to normal skin. Total RNA samples from punch biopsies of five acne lesions and matched non-lesional controls (normal skin) were obtained from D. Thiboutot15. mRNA expression profiles were assessed using Affymetrix gene expression microarray system. For each of the donors, G2A mRNA expression (GPR132) was higher in the acne lesion biopsies than in the matched controls. When the expression values of donors were averaged, the mean GPR132 expression was doubled (Fig. 5A, p<0.001) in acne lesions compared to normal skin. Comparison of secretoglobin SCGB1D2 (p<0.01) and human beta-defensin 4 DEFB4 (p<0.05) from the current data set corroborates previously published microarray data obtained from the same lesional and control samples using a previous version of the Affymetrix human array (U133A 2.0)15.
Retinoids, a class of vitamin A-derived compounds that bind various members of the retinoic acid receptor181920, affect keratinocyte maturation and anti-inflammatory activity2122. Retinoids also downregulate TLR2 expression in monocytes23. We investigated whether 13cRA and ATRA, two widely used therapeutics for acne2425 could regulate G2A expression in monocytes. Monocytes were pre-treated with 13cRA and ATRA for 1 hour prior to a 24 hours exposure to live PA. These monocytes were then labeled with anti-G2A antibodies and analyzed using flow cytometry. Compared to control, ATRA alone did not induce a significant change in the percentage of cells expressing G2A but 13cRA induced a 10-fold increase (Fig. 5B, p<0.001). When exposed to PA, monocytes exhibited a 100-fold increase in the percentage of cells expressing G2A compared to untreated cells (p<0.0001). Concurrent treatment with ATRA did not significantly modulate the PA-induced upregulation of G2A whereas 13cRA increased G2A expression on monocytes by approximately 30% compared to PA alone (p<0.05).
The active disease site of acne is the pilosebaceous unit, a lipid-rich environment where the commensal microbe PA colonizes and can induce inflammation. While increased sebum production is observed in acne, the mechanism by which lipids play a role in pathogenesis remains unclear. G2A provides one model that illustrates how lipid receptors may attenuate inflammation to a bacterium and regulate immune response to normal skin microflora.
We studied G2A in response to PA in human monocytes given previous studies showing relatively high levels of G2A monocyte expression and that monocytes are consistently used to study inflammatory responses to PA5172627. First, we found that both G2A is inducible by PA and may potentiate an anti-inflammatory cascade, as we see through our G2A knockdown model leading to upregulation of pro-inflammatory cytokines: IL-6, IL-8, and IL-1β, markers associated with PA-induced inflammation28. These cytokines play pivotal roles in host defense29, neutrophil signaling, lysosomal pathways30, and early innate immune responses to bacterial pathogens31.
G2A has high affinity for the stable oxidized linoleic acid derivative, 9-HODE17. We show that the G2A ligand, 9-HODE, is also found in higher concentrations from monocytes in response to PA, presumably through an oxidative process, thus introducing a possible pathway by which oxidative changes to FFAs modulate downstream inflammatory responses. The inflammatory properties of 9-HODE has not been characterized in skin. However, there are studies from the atherosclerosis model that show that these lipids play a role in regulating monocyte function, such as retaining monocytes at the sites of inflammation3233. We acknowledge, however, that our findings can only suggest a possible autocrine or paracrine response among monocytes and does not completely model the interaction between G2A and 9-HODE from sebum and resident skin cells.
In response to PA exposure, G2A response appears connected to innate immune pathways as its effects are partially mediated by TLR2, which is sufficient for the activation of NF-κB in response to PA exposure required for the later downstream pro-inflammatory cytokine promoter activity17. We observed a significant 30% reduction in G2A expression in monocytes treated with TLR2 neutralizing antibody as compared to cells pretreated with IgG isotype control. Furthermore, TLR downstream signaling pathways ERK1/2 MAPK and NF-κB p65, but not the p38 pathway, was reduced following PA simulation in G2A-knockdown monocytes. This discrepancy of MAP kinase pathway activation is also seen in other models34, such as in osteoblasts and corneal epithelial35. One possible explanation for this bifurcation may be due to the known differences of p44/42 versus p38 MAP kinase pathways—e.g., the p44/42 isoforms of MAP kinase are more associated with growth factors and GPCRs. The question of whether there is crosstalk between G2A, a GPCR, and TLR2; or whether these two receptors modulate activation of NF-κB and MAP kinase independent of one another remains to be elucidated. However, there is a growing body of knowledge that shows how GPCRs and TLRs can exhibit reciprocal activity by regulating the signaling of each other36.
By looking at the effects of retinoic acids on G2A and analyzing RNA samples from acne lesions, we sought to associate G2A to the clinical setting. One of the desired outcomes of isotretinoin treatment is a reduction of inflammation by a mechanism incompletely understood3738. Given that isotretinoin induced higher expression of G2A in monocytes, and attenuated pro-inflammatory cytokines IL-6 and IL-8, this mechanism may suggest that this reduction in inflammation clinically may be associated with its effect on G2A3639404142. Our findings that G2A is upregulated in monocytes in response to PA was mirrored in acne lesion punch biopsies compared to matched normal skin. While acne lesions are characterized by increased inflammation, it is known that there are concurrent anti-inflammatory and anti-microbial pathways that are activated during disease, such as through antimicrobial peptides and IL-104344. While our skin biopsies contain a milieu of both resident skin cells and immune cells, the general upregulation of G2A in the skin as well as in monocytes may act as one of these anti-inflammatory pathways.
Our main goals in studying G2A were to define a possible mechanism by which lipids mediate inflammation in response to the PA and to further elucidate the immune regulatory roles of this receptor, especially in light of recent findings that G2A may be important in regulating immunity to commensal bacteria. Yet an important question that remains is whether G2A expression correlates with acne severity. As Cohen et al.7 showed that G2A may play a role in controlling immune response to gut commensal bacteria, we can speculate that patients with severe acne may either have dysregulated expression of G2A in response to PA colonization or that the effects of G2A in acne patients may be an appropriate response to commensal bacterium in the context of acne disease driven by other inflammatory pathways. Furthermore, while we were able to demonstrate a global upregulation of G2A expression in acne lesions, because of the inherent difficultly of obtaining acne lesion biopsies, our study was limited in its ability to relate G2A clinically. Rather, the scope of this study focused on the molecular mechanisms by which G2A may mediate innate immune response to PA and introduce a mechanism by which lipids may mediate inflammatory in acne pathogenesis.
In summary, we show that G2A is upregulated in acne lesions, and both G2A and its ligand are upregulated by PA and may play an important anti-inflammatory function that quells pathogenesis to a commensal bacterium. Here we also introduce a possible pathway by which acne pathogenesis is regulated by lipid mediators and help further understand the immune functions of G2A.
Figures and Tables
 | Fig. 1Propionibacterium acnes (PA), but not 9-hydroxyoctadecadienoic acid (9-HODE), upregulates G2A in monocytes via a Toll-like receptor 2 (TLR2)-dependent mechanism. (A) Quantitative polymerase chain reaction (qPCR) and (B) fluorescence-activated cell sorting (FACS) analysis revealed that PA, but not 9-HODE, upregulates both the mRNA expression and the population of cells expressing G2A in both the THP-1 cell line and in primary human monocytes. Values from PCR are shown as mean±standard error (SE) (n=4) and FACS data is representative of three independent experiments. (C) Blocking antibody to TLR2 neutralized the upregulation of G2A mRNA expression by PA in monocytes. Values are expressed as mean±standard error (n=3). MOI: multiplicity of infection, IgG: immunoglobulin G. *p<0.05, **p<0.01. |
 | Fig. 2Induction of 9-hydroxyoctadecadienoic acid (9-HODE) in human monocytes by Propionibacterium acnes (PA). Mass spectrometry analysis of G2A ligand and linoleic acid derivative, 9-HODE, and arachidonic acid derivative, 15-hydroxyeicosatetraenoic acids, in human monocytes by PA. Values are expressed as mean±standard error and were compiled from three independent experiments. MOI: multiplicity of infection. *p<0.05, **p<0.001. |
 | Fig. 3Effects of G2A knockdown on interleukin (IL)-6, IL-8, and IL-1β cytokine secretion and gene expression. The secreted protein and mRNA from G2A knockdown THP-1 cells were analyzed using enzyme-linked immunosorbent and quantitative polymerase chain reaction. (A) G2A knockdown enhanced IL-6 secretion in response to Propionibacterium acnes (PA) and (B) upregulation of IL6 gene expression in response to PA and 9-hydroxyoctadecadienoic acid (9-HODE). (C) G2A knockdown enhanced IL-8 response to PA and 9-HODE both at the protein level and the gene (D) level. (E) G2A knockdown had no measureable effect on IL-1β cytokine response to PA or 9-HODE, (F) but enhanced IL-1β response to PA and 9-HODE at the gene level. Values are expressed as mean±standard error (n=4). siRNA: small interfering RNA. *p<0.05, **p<0.01, ***p<0.001. |
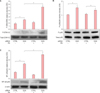 | Fig. 4Effects of G2A knockdown on ERK1/2 and nuclear factor kappa B (NF-κB) p65 activation. (A~C) Western blot studies on human monocytes using an anti-G2A small interfering RNA (siRNA). G2A was partially knocked down in human monocytes using an anti-G2A siRNA. These cells were then analyzed using western blot to measure differences in activation of select molecular pathways in response to Propionibacterium acnes (PA). *p<0.05. |
 | Fig. 5G2A mRNA expression in acne lesions and human monocytes. (A) Assessment of RNA isolated from acne lesions and matched normal skin revealed an increase in the gene expression for G2A, GPR132. Also shown are the negative and positive controls, SCGB1D2 and DEFB4, respectively. Values connected by points are from individual donors and values expressed by a bar are mean values (n=5). (B) G2A expression in human monocytes in response to Propionibacterium acnes, all-trans-retinoic acid (ATRA), and 13-cis-retinoic acid (13cRA) as measured by fluorescence-activated cell sorting analysis. Values are expressed as percentage of cells expressing G2A (n=3). *p<0.05, **p<0.01, ***p<0.001. |
ACKNOWLEDGMENT
This work was supported by the NIH NIAMS R01 AR053542, an Annenberg Foundation Research Grant, and an American Skin Association Research Grant to JK; a LEO Pharma Research Grant to MQ; and an NIH T32 Scientist Training Grant to GA. We would also like to thank Drs. Greg Hough and Mohamad Navab for their invaluable assistance and guidance.
References
1. Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, et al. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009; 284:12328–12338.

2. Obinata H, Hattori T, Nakane S, Tatei K, Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005; 280:40676–40683.

3. Downing DT, Stewart ME, Wertz PW, Strauss JS. Essential fatty acids and acne. J Am Acad Dermatol. 1986; 14:221–225.

4. McGinley KJ, Webster GF, Ruggieri MR, Leyden JJ. Regional variations in density of cutaneous propionibacteria: correlation of Propionibacterium acnes populations with sebaceous secretion. J Clin Microbiol. 1980; 12:672–675.

5. Bolick DT, Whetzel AM, Skaflen M, Deem TL, Lee J, Hedrick CC. Absence of the G protein-coupled receptor G2A in mice promotes monocyte/endothelial interactions in aorta. Circ Res. 2007; 100:572–580.

6. Hattori T, Obinata H, Ogawa A, Kishi M, Tatei K, Ishikawa O, et al. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol. 2008; 128:1123–1133.

7. Cohen LJ, Kang HS, Chu J, Huang YH, Gordon EA, Reddy BV, et al. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc Natl Acad Sci U S A. 2015; 112:E4825–E4834.

8. Pappas A, Anthonavage M, Gordon JS. Metabolic fate and selective utilization of major fatty acids in human sebaceous gland. J Invest Dermatol. 2002; 118:164–171.

9. Greene RS, Downing DT, Pochi PE, Strauss JS. Anatomical variation in the amount and composition of human skin surface lipid. J Invest Dermatol. 1970; 54:240–247.

10. Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, Huang CM. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol. 2010; 130:985–994.

11. Choi CW, Choi JW, Park KC, Youn SW. Facial sebum affects the development of acne, especially the distribution of inflammatory acne. J Eur Acad Dermatol Venereol. 2013; 27:301–306.

12. Vromman F, Subtil A. Exploitation of host lipids by bacteria. Curr Opin Microbiol. 2014; 17:38–45.

13. Schenk M, Krutzik SR, Sieling PA, Lee DJ, Teles RM, Ochoa MT, et al. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med. 2012; 18:555–563.

14. Imaizumi S, Grijalva V, Navab M, Van Lenten BJ, Wagner AC, Anantharamiah GM, et al. L-4F differentially alters plasma levels of oxidized fatty acids resulting in more anti-inflammatory HDL in mice. Drug Metab Lett. 2010; 4:139–148.

15. Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. 2006; 126:1071–1079.

16. Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013; 339:1448–1453.

17. Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002; 169:1535–1541.

18. Krutzik SR, Sieling PA, Modlin RL. The role of Toll-like receptors in host defense against microbial infection. Curr Opin Immunol. 2001; 13:104–108.

19. Leyden JJ. A review of the use of combination therapies for the treatment of acne vulgaris. J Am Acad Dermatol. 2003; 49:3 Suppl. S200–S210.

20. Okuno M, Kojima S, Matsushima-Nishiwaki R, Tsurumi H, Muto Y, Friedman SL, et al. Retinoids in cancer chemoprevention. Curr Cancer Drug Targets. 2004; 4:285–298.

21. Chalker DK, Lesher JL Jr, Smith JG Jr, Klauda HC, Pochi PE, Jacoby WS, et al. Efficacy of topical isotretinoin 0.05% gel in acne vulgaris: results of a multicenter, double-blind investigation. J Am Acad Dermatol. 1987; 17:251–254.

22. Gibbs S, Backendorf C, Ponec M. Regulation of keratinocyte proliferation and differentiation by all-trans-retinoic acid, 9-cis-retinoic acid and 1,25-dihydroxy vitamin D3. Arch Dermatol Res. 1996; 288:729–738.

23. Dispenza MC, Wolpert EB, Gilliland KL, Dai JP, Cong Z, Nelson AM, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012; 132:2198–2205.

24. Wojtal KA, Wolfram L, Frey-Wagner I, Lang S, Scharl M, Vavricka SR, et al. The effects of vitamin A on cells of innate immunity in vitro. Toxicol In Vitro. 2013; 27:1525–1532.

25. Saxon A, Keld B, Braun J, Dotson A, Sidell N. Long-term administration of 13-cis retinoic acid in common variable immunodeficiency: circulating interleukin-6 levels, B-cell surface molecule display, and in vitro and in vivo B-cell antibody production. Immunology. 1993; 80:477–487.
26. Qin M, Pirouz A, Kim MH, Krutzik SR, Garbán HJ, Kim J. Propionibacterium acnes Induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014; 134:381–388.

27. Liu PT, Phan J, Tang D, Kanchanapoomi M, Hall B, Krutzik SR, et al. CD209(+) macrophages mediate host defense against Propionibacterium acnes. J Immunol. 2008; 180:4919–4923.

28. Aarbiou J, Verhoosel RM, Van Wetering S, De Boer WI, Van Krieken JH, Litvinov SV, et al. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol. 2004; 30:193–201.

30. Webster GF, Leyden JJ, Tsai CC, Baehni P, McArthur WP. Polymorphonuclear leukocyte lysosomal release in response to Propionibacterium acnes in vitro and its enhancement by sera from inflammatory acne patients. J Invest Dermatol. 1980; 74:398–401.

31. Mastrofrancesco A, Kokot A, Eberle A, Gibbons NC, Schallreuter KU, Strozyk E, et al. KdPT, a tripeptide derivative of alpha-melanocyte-stimulating hormone, suppresses IL-1 beta-mediated cytokine expression and signaling in human sebocytes. J Immunol. 2010; 185:1903–1911.

32. Han KH, Chang MK, Boullier A, Green SR, Li A, Glass CK, et al. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor gamma. J Clin Invest. 2000; 106:793–802.

33. Pankonin G, Mahmood SA, Kuhn H, Schewe T, Pilgrim H, Teuscher E. Inhibition of cell migrations by the linoleic acid oxygenation product 9S-hydroxy 10E,12Z octadecadienoic acid (9-HODE). Biomed Biochim Acta. 1988; 47:K17–K21.
34. Eda H, Shimada H, Beidler DR, Monahan JB. Proinflammatory cytokines, IL-1β and TNF-α, induce expression of interleukin-34 mRNA via JNK- and p44/42 MAPK-NF-κB pathway but not p38 pathway in osteoblasts. Rheumatol Int. 2011; 31:1525–1530.

35. Nakamura M, Chikama T, Nishida T. Participation of p38 MAP kinase, but not p44/42 MAP kinase, in stimulation of corneal epithelial migration by substance P and IGF-1. Curr Eye Res. 2005; 30:825–834.

36. Shi GX, Harrison K, Han SB, Moratz C, Kehrl JH. Toll-like receptor signaling alters the expression of regulator of G protein signaling proteins in dendritic cells: implications for G protein-coupled receptor signaling. J Immunol. 2004; 172:5175–5184.

37. Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest. 2008; 118:1468–1478.

38. Harper JC, Thiboutot DM. Pathogenesis of acne: recent research advances. Adv Dermatol. 2003; 19:1–10.
39. Strauss JS, Stranieri AM. Changes in long-term sebum production from isotretinoin therapy. J Am Acad Dermatol. 1982; 6(4 Pt 2):Suppl. 751–756.

40. King K, Jones DH, Daltrey DC, Cunliffe WJ. A double-blind study of the effects of 13-cis-retinoic acid on acne, sebum excretion rate and microbial population. Br J Dermatol. 1982; 107:583–590.

41. Landthaler M, Kummermehr J, Wagner A, Plewig G. Inhibitory effects of 13-cis-retinoic acid on human sebaceous glands. Arch Dermatol Res. 1980; 269:297–309.

42. Goldstein JA, Comite H, Mescon H, Pochi PE. Isotretinoin in the treatment of acne: histologic changes, sebum production, and clinical observations. Arch Dermatol. 1982; 118:555–558.

SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-29-688-s001.pdf.
Supplementary Fig. 1
Efficiency of GPR132 knockdown using small interfering RNA (siRNA). Transfection of THP-1 cells using siRNA and electroporation consistently yielded at least a 50% knockdown of GPR132 mRNA expression measured by quantitative polymerase chain reaction when compared to the control siRNA.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download