INTRODUCTION
Twenty-nail dystrophy (TND) of childhood was first used by Hazelrigg et al.
1 to describe an idiopathic nail dystrophy in six children, which began insidiously in early childhood, and was characterized by longitudinal ridging and roughness. TND as also been referred to as ‘trachyonychia’, a term that is well suited to the clinical appearance of the affected nails
234. The quality of life of patients with TND is generally decreased due to the resulting deformation of nails. The exact etiology of TND remains unclear.
Many treatment modalities are used in order to prevent nail destruction and reduce resolving duration. Therapeutic modalities such as topically-applied psoralen-ultra violet A (PUVA), triamcinolone acetonide injections, topical steroid, and systemic agents include prednisolone, anti-malarials, and etretinate in TND have been tried
5678910111213. However, these treatments have low efficacies. In addition, injections are painful and thus are difficult to administer to children. Thus, a standard treatment for TND has not yet been established.
Cyclosporine is a common therapeutic agent that selectively inhibits T-cell activation, has been successfully used for various skin diseases and continues to play an important role in the field of dermatology
1415. Although one recent study has reported the effects of low-dose cyclosporine in 15 patients with TND, no large-scale trial has yet been conducted
16. We estimated that cyclosporine reduces spongiotic dermatitis of nail matrix by inhibiting T cell activation in TND. And pantothenic acid complex-based dietary supplement (Pantogar®; Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) is used as a dietary supplement that helps nail growth
17. Combination therapy commonly has been done when treatment effect of monotherapy is not enough. Therefore we used combination therapy based on the two above mechanisms.
The aim of the present study was to evaluate the effectiveness of oral cyclosporine in patients with TND by comparing combination therapy of cyclosporine and pantothenic acid complex-based dietary supplement and pantothenic acid complex-based dietary supplement alone.
Go to :

DISCUSSION
TND is usually idiopathic, i.e., it may occur without any associated dermatological disease
2. TND may be associated with various dermatological conditions including lichen planus, psoriasis, and alopecia areata, rare associations with vitiligo and incontinentia pigmenti have also been reported
20212223. In our study, we focused on idiopathic TND that is not associated with any other dermatological condition. Inflammation of the nail matrix may produce TND. Most patients with TND show spongiotic dermatitis, with mild inflammation associated with lymphocytic exocytosis of the proximal nail fold and proximal nail matrix. Cyclosporine has a selective effect on T-cell proliferation by inhibiting the synthesis of interleukin-2
14. Cyclosporine acts primarily as a systemic immune modulator by binding cyclophilin and blocking calcineurin
24. Cyclosporine is currently used by dermatologists in the management of psoriasis and atopic dermatitis. The therapeutic efficacy of cyclosporine in TND has been reported previously
16.
Lee et al.
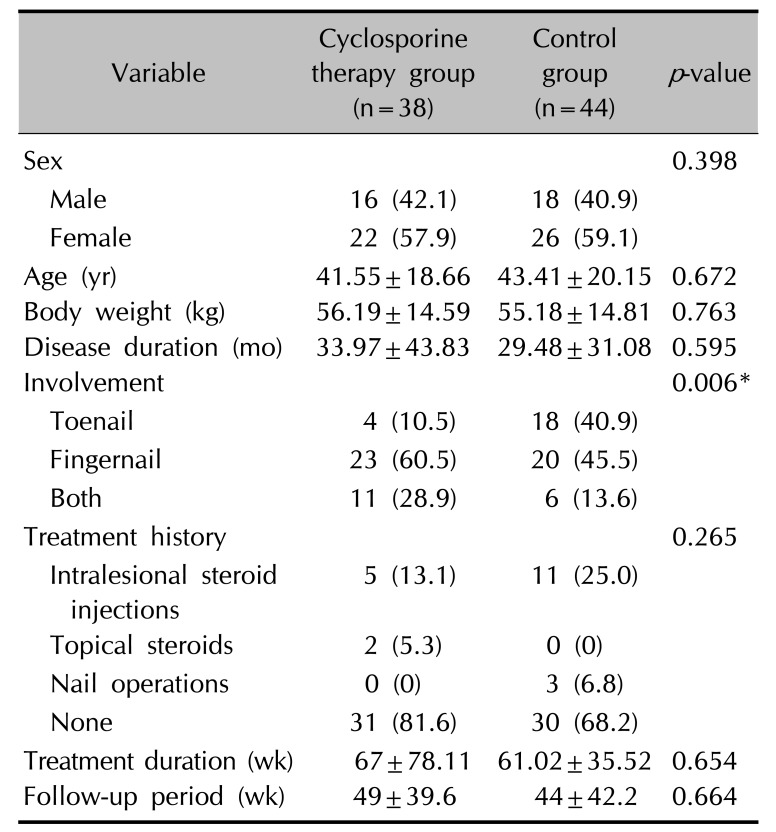
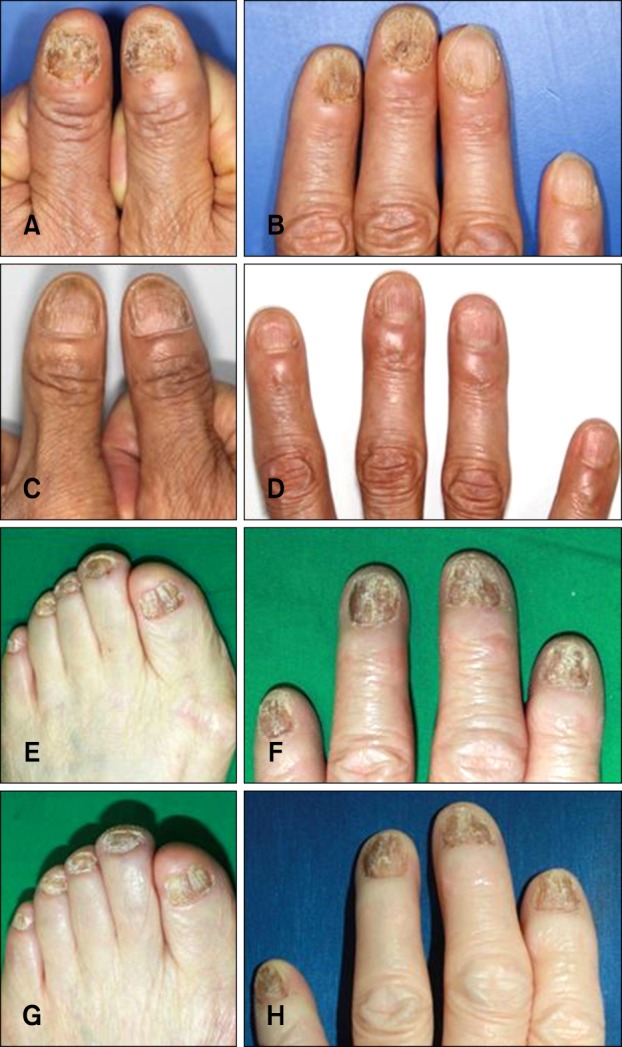
16 reported that low dose cyclosporine administration (2.0~3.5 mg/kg per day) may be beneficial in the management of TND and in improving quality of life. In the above study, the clinical improvement was evaluated by using the clinical photos. The evaluation was classified as complete resolution, significant improvement (>50% improvement of fingernail dystrophy), slight improvement (<50% improvement), or no change. All patients with TND (n=15) presented with slight improvement of fingernail plate roughness after one month. After 6 months of treatment, 13 patients (86.7%) showed significant improvement of TND and two patients maintained slight improvement (13.3%). The mean pretreatment DLQI score was 18.1. After treatment, the mean DLQI score was significantly decreased to 9.5. However, the above study has small sample size and no comparable control group was included. Furthermore, the above study only evaluated clinical improvement of fingernails, and excluded toenails. In the present study, to evaluate the effectiveness of cyclosporine in patients with TND, a total of 38 patients with TND were treated with combination therapy using oral cyclosporine with Pantogar® and 44 patients were treated with Pantogar®. The therapeutic efficacy in each group was retrospectively evaluated using medical records and clinical photographs. The pretreatment to post-treatment DLQI change was greater in the cyclosporine therapy group (
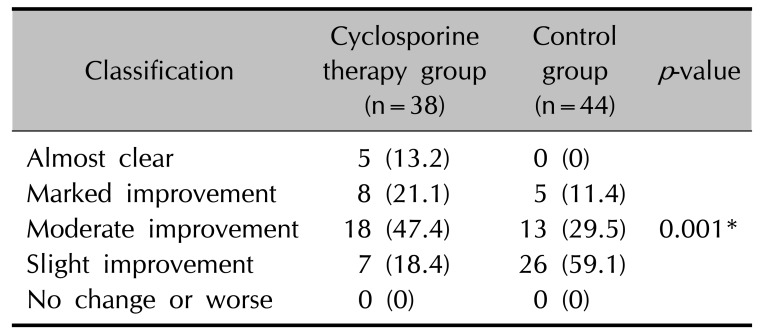
p=0.085). This result demonstrates that cyclosporine administration may be beneficial in improving the quality of life in patients with TND. The cyclosporine therapy group had more patients who were almost clear of nail roughness or whose improvement was rate as marked or moderate compared to the control group (
p=0.001). The mean CHATS score in the cyclosporine therapy group decreased by 13.45 after treatment, but decreased by only 8 in control group. This difference was statistically significant (
p<0.001). No severe adverse reactions to cyclosporine were observed in this study. Most of patients were satisfied about the clinical improvement after 6 months of treatment. Referencing the above out result, authors recommend that administrate 3~5 mg/kg cyclosporine per day in TND for about 6 months or longer.
We hypothesize that the therapeutic effectiveness of cyclosporine in TND results from its dramatic effects on T-cell function, as well as on pro-inflammatory cytokine release. In this manner, cyclosporine might suppress the spongiotic dermatitis and lymphocytic infiltration of the nail matrix observed in patients with TND.
The adverse effects of cyclosporine are, for the most part, dose-dependent and related to therapy duration
25. Gastrointestinal discomfort is a common side effect of cyclosporine administration. Cyclosporine is contraindicated in patients with uncontrolled hypertension, renal disease, serious infections, and a previous history of malignancy, excluding basal cell carcinoma
25. In our study, we excluded patients with any of the above diseases. Moreover, laboratory abnormalities or severe adverse reactions were not observed during the cyclosporine treatment period. Four of the 38 patients (10.53%) reported nausea and abdominal discomfort; however, these symptoms were mild and were alleviated by taking digestive medication. Using the recommended monitoring protocols has been shown to result in a significant decrease in the incidence of cyclosporine-related toxicities
25.
Although this is the first report to assess the treatment efficacy of cyclosporine in patients with TND and to use a control group, there are some limitations to our study. First, the TND nail severity score may be less accurate due to use of a nail severity scoring system for onychomycosis. Second, our results may be subject to recall bias, since the pretreatment DLQI score was estimated retrospectively after treatment. Third, the studied population was from a small geographic area and thus our results may not be generalizable to a larger population. Fourth, our study was performed at a single-center, meaning that it may subject to selection bias. It was also a retrospective study and there may have been multiple confounding factors for which could not control. Future large, randomized, controlled multicenter trials are required to validate the correlations we report here.
In conclusion, our results show that cyclosporine is an effective treatment option for TND. Since few studies have assessed TND treatments, our study is of note in that it was conducted using a relatively large number of patients with TND. Furthermore, our study is a milestone because few studies have reported the effects of cyclosporine in TND.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download