INTRODUCTION
Varicella results from primary infection with varicella-zoster virus (VZV), and herpes zoster is caused by the reactivation of latent VZV in the sensory ganglia
1. The mechanisms underlying the reactivation of latent VZV are unclear. However, reactivation is related to immunodeficiency, irradiation of the spinal column, and local trauma, and the most important factor for reactivation is decreased VZV-specific cell-mediated immunity with advancing age
1. A recent study confirmed that VZV-specific T cells in the peripheral blood decrease with advancing age, whereas regulatory T (Treg) cells increase
2. T cell-mediated immunity may play an important role in the reactivation of VZV via changes in CD4+ T cells and cytotoxic CD8
+ T cells after the development of herpes zoster
34.
Tissue injury in common viral infections can be directly caused by viral replication or immunopathology; it also occurs as a result of an increased inflammatory response associated with viral replication or the inhibition of the inflammatory response to the virus
5. Treg cells in viral infections can control anti-viral inflammation and prevent immunopathology, but they inhibit antiviral immunity, facilitate viral replication, and cause persistent viral infections
5. T-helper 17 (Th17) cells play an important role in the host defense against some viral infections, but they can cause detrimental immunopathological responses
5. A number of studies have examined the roles of Treg cells and Th17 cells in chronic viral diseases, such as chronic hepatitis B and C virus infections, and have shown that the relationship between Treg cells and Th17 cells, rather than each of the T cell subsets alone, may play an important role in disease progression and virus persistance
67. Although recent studies have examined changes in Treg cells in the peripheral blood and postherpetic neuralgia (PHN) in patients with herpes zoster, no study has evaluated the correlation between Treg cells and Th17 cells. In the present study, Treg cells, Th17 cells, and the Th17/Treg cell ratio were examined in the peripheral blood from patients with herpes zoster and normal controls using flow cytometry to investigate their correlations with the pathogenesis of herpes zoster.
Go to :

MATERIALS AND METHODS
Subjects
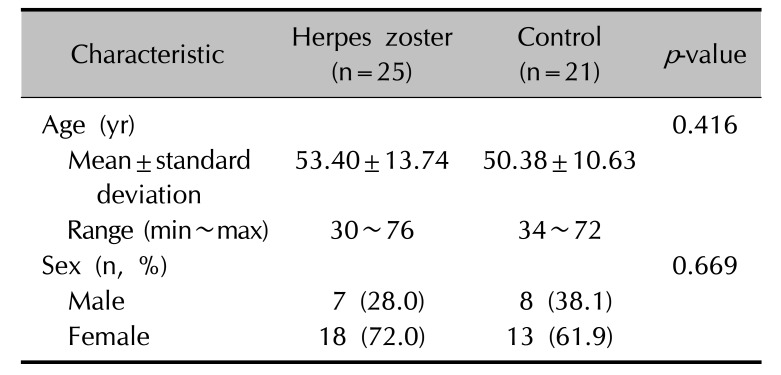
The subjects consisted of 25 patients with herpes zoster, including 13 inpatients who visited the Dermatology Department outpatient care clinic at this hospital and were diagnosed with herpes zoster from September 2015 to March 2016 and 21 healthy controls recruited during the same period. The present study was approved by the Institutional Review Board of Chosun University Hospital, Gwangju, Korea (IRB no. CHOSUN 2015-07-013-001). All subjects agreed to participate in the study. Patients were excluded if they were taking any antiviral drug prescribed at another hospital prior to their visit to this hospital, if they took any anti-inflammatory analgesic drug for the purpose of pain control prior to treatment, and if they were lost to follow-up. Controls with similar age and gender distributions were recruited among general individuals who did not have herpes zoster, received no vaccination against herpes zoster within one year, and had no past history or family history of autoimmune diseases, as identified in an interview.
Methods
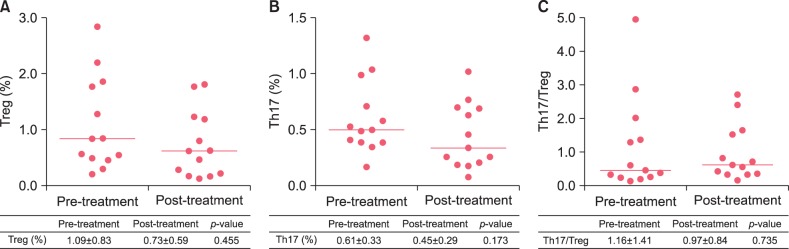
Blood was collected once from all subjects before treatment, and blood was collected one additional time from the hospitalized patients after treatment for 5 days or before discharge from the hospital to measure changes in Treg and Th17 cells before and after treatment. The hospitalized patients received treatment with acyclovir at a dose of 5 mg/kg intravenously three times daily for 5 days with analgesics and didn't take any systemic agents including systemic glucocorticosteroid that may affect immune cells. The affected body surface area (BSA) was measured using clinical photographs obtained at admission. Pain scores before and after treatment were assessed on a scale from 0 (no pain) to 10 points (the most severe pain) in patients with herpes zoster using the visual analog scale (VAS). PHN was defined as a pain index of greater than 30% of the initial pain even after 4 weeks of skin rash
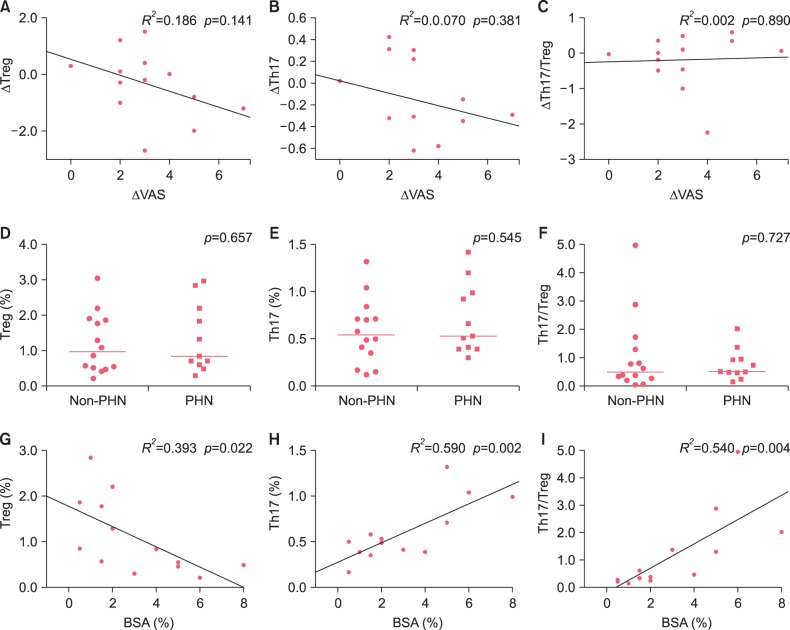
8, and the development of PHN was examined after 4 weeks of hospital treatment. The proportions of Treg cells and Th17 cells and the Th17/Treg ratio were compared in patients with herpes zoster before and after treatment to evaluate the effects of treatment. The relationships between the proportion of each of T cell subset in the peripheral blood and herpes zoster-related clinical parameters, such as disease duration, pain scores, presence or absence of PHN, and affected area of skin (BSA), were evaluated.
Cell isolation
Venous blood (10 ml per subject) was collected from patients with herpes zoster and normal controls in heparin-treated tubes. Ficoll-Paque™ PLUS (10 ml) (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) was added to a 50-ml tube and the blood was then carefully added to the top of Ficoll-Paque™ PLUS, followed by centrifugation for 40 min (400×g, 20℃). After centrifugation, 3 ml of the peripheral blood mononuclear cell (PBMC) fraction was pipetted using a micropipette, and was transferred to a 15-ml tube, mixed thoroughly with phosphate-buffered saline, and washed twice (200×g, 10 minutes, 20℃) to prepare PBMCs.
Flow cytometric analysis
Th17 cells were defined as helper T cells that produce interleukin (IL)-17. The IL-17 Secretion Assay-Detection Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) was used to determine the number of cells that produce IL-17. Two samples of 5×106 PBMCs cultured in 500 µl of RPMI 1640 with 5% human serum were prepared according to the manufacturer's protocol, and each sample was added to a 48-well plate. T cells were stimulated with CytoStim® (Miltenyi Biotec) (10 µl) in one 48-well plate, and were cultured in a 5% CO2 incubator at 37℃ for 4 hours. CytoStim® induces the activation of T cells. Activated CD4+ T cells may secrete effector cytokines within several hours or begin to express activation markers on cell surfaces. After cultivation, each sample was stained with monoclonal antibodies against CD4-fluorescein isothiocyanate (FITC) and IL-17-allophycocyanin (APC). The sample without CytoStim® was treated as the negative control, and the proportions of CD4+IL-17+ T cells relative to CD4+ cells were then converted to percentages using the Navios Flow Cytometer (Beckman Coulter, Krefeld, Germany) to quantify T cell subsets.
Treg cells were defined as CD4+CD25+Foxp3+ T cells. In order to determine Treg cells, Treg cell surfaces were stained for cell surface antigens of PBMCs using CD4-FITC and CD25-APC (Miltenyi Biotec) monoclonal antibodies. Then, cells were subjected to fixation and permeabilization processes. Subsequently, intracellular staining was performed with Anti-Foxp3-PE (phycoerythrin; Miltenyi Biotec). Stained cells were analyzed by three-color fluorescence activated cell sorting (FACS) using a flow cytometer and Kaluza Analysis Software (Beckman Coulter, Brea, CA, USA), and the proportions of CD4+CD25+Foxp3+ T cells relative to total CD4+ cells were converted to percentages to obtain the number of Treg cells.
The ratios of Th17 cells to Treg cells were calculated to investigate the relationship between Th17 and Treg cells in the development and treatment of herpes zoster.
Statistical analyses
Mann-Whitney U test (Wilcoxon rank sum test), Wilcoxon signed rank test, Student's t-tests and χ2–test were used to evaluate differences in Th17 cells, Treg cells, and Th17/Treg cell ratios between herpes zoster and control. Their relationships with various clinical parameters in patients with herpes zoster before and after treatment and controls were analyzed using Pearson's correlation tests. All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Graphs were plotted using GraphPad Prism 7.02 software. A p-value of less than 0.05 was statistically significant.
Go to :

DISCUSSION
Herpes zoster caused by the reactivation of VZV is characterized by grouped vesicles accompanied by severe pain, and PHN, i.e., pain persisting for several months or years after the rash has resolved, can develop as a consequence
910. The cellular mechanisms of the reactivation of the latent virus and the precise causes of PHN are unclear
3. However, in view of the fact that treatments or diseases that affect the functions of T cells increase the risk of herpes zoster and HIV-infected patients with a progressive decrease in CD4
+ cells are at high risk for herpes zoster, cell-mediated T cells are thought to play an important role in the development of herpes zoster
39.
Naïve CD4
+ T cells can differentiate into Th1, Th2, Treg, and Th17 cells under certain conditions and produce a variety of cytokines that mediate immune responses
11. Transforming growth factor (TGF)-β induces the differentiation of naïve CD4
+ T cells into Treg cells to produce TGF-β and IL-10, maintain immunological tolerance, and control inflammatory responses. TGF-β and IL-6 induce the differentiation of naïve CD4
+ T cells into Th17 to produce IL-17, -21, and -23 and promote inflammatory responses
11. Treg differentiation and Th17 differentiation are related processes; the two T cell subsets play an important role in maintaining immunological homeostasis and there may exist an important plasticity of the two T cell subsets
7.
A recent study has reported that during viral infection, CD4
+CD25
+ Treg cells inhibit the activation and functions of effector T cells and, in turn, control antiviral responses
3. Tissue damage in viral infection can be caused directly by viral propagation in the tissue, and this is regulated by the antiviral immune response, which acts to suppress viral replication
5.
Although Treg cells can be beneficial in viral infections by acting to suppress tissue damage caused by virus-specific T cells, Treg cells can suppress host immunity, which is instrumental in eradicating viruses, leading to persistent viral infection and disease progression
1213. CD4
+ cells and CD8
+ cytotoxic cells play an important role in controlling VZV replication in the ganglia during the acute phase of herpes zoster
4. CD8
+ cytotoxic T cells play a key role in the control of intracellular viral replication by eliminating infected cells. When cytotoxic T cell responses are impaired or the virus alters cytotoxic responses, a chronic viral infection can result. Treg cells are also involved in the maintenance of a persistent viral infection
512. In a study on herpes zoster-related antigens, Treg cells increased in the peripheral blood and skin tissue with increasing age; when the skin was sensitized to the VZV antigen, CD4
+Foxp3
+ T cells increased and there was an inverse correlation between CD4
+Foxp3
+ T cells and VZV skin reactions. Thus, CD4
+Foxp3
+ T cells were thought to suppress antigen-specific responses
14.
Th17 cells that produce IL-17 are associated with inflammatory tissue damage, lead to a variety of autoimmune diseases, and play a key role in host defense against bacterial infections or fungal infections
15. Th17 cells in viral infections generally cause immunopathology, with detrimental effects on the host
15. IL-17 secreted by Th17 cells suppresses the production of IL-2 and interferon-γ and, in turn, inhibits the differentiation of Th1 cells, which have cytotoxic and antiviral functions, thus causing persistent viral infections
515. If Th17 cells suppress Th1 immune responses, viral replication cannot be controlled or it can be detrimental to the host by mediating immunopathology
12. Although Th17 cells induce immunopathological responses in some infections, they are essential and protective in the host response to intracellular bacteria or viruses
5. Deficiencies in Th17 cells caused by viral infections, such as HIV infection, can exacerbate diseases caused by extracellular bacteria or viruses, and lead to increased opportunistic infections
5. In 2016, Zajkowska et al.
16 first reported that Th17-related cytokines (IL-17, 21, 23) levels were significantly higher in herpes zoster than controls. However, they stated that further studies are needed to investigate that. This result indirectly suggests that Th17 cells may be high in herpes zoster.
An imbalance between Treg cells and Th17 cells is associated with the progression or prognosis of autoimmune diseases, such as psoriasis and atopic dermatitis, or inflammatory diseases. Recently, studies have examined the imbalance between the two T cell subsets in chronic viral diseases, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) infection
6711. According to a study by Xue-Song et al.
6, Th17 cells are involved in acute and chronic HBV infection, and an imbalance in the Treg/Th17 ratio was observed for chronic hepatitis B and acute-on-chronic HBV-related liver failure patients, which was linked to disease progression and continuous HBV infection. In addition, in a study of chronic HCV-infected patients by Hao et al.
7, Treg cell proportions were significantly elevated in HCV-infected patients, but there was no significant difference in the proportion of Th17 cells between infected and uninfected individuals. Furthermore, when HCV replication was inhibited, there was a reduction in Treg cells, leading to a significant decrease in the Treg/Th17 ratio. An imbalance in the ratio of Treg cells to Th17 cells might play an important role in persistent HCV infection. Herpes zoster may have acute and chronic responses, because of acute viral infection and chronic exposure of VZV antigen during periodic episodes of subclinical reactivation.
In a recent study of Treg cells in patients with herpes zoster by Xing et al.
3, the number of CD4
+CD25
+Foxp3
+ T cells in the peripheral blood of patients with herpes zoster was significantly higher than that of normal controls and tended to increase as pain increased in severity. Furthermore, the proportions of CD4
+CD25
+Foxp3
+ T cells were significantly higher in the group with PHN than the group without PHN. These findings suggest that T cell immunity was impaired in patients with herpes zoster, and increased activation of Treg cells might suppress the antiviral immune response of CD4
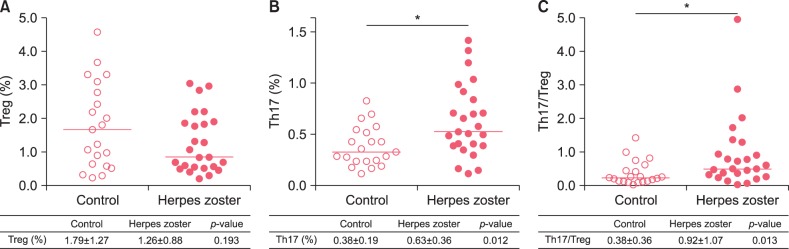
+ T cells. Accordingly, they asserted that Treg cells may play an important role in the pathogenesis of herpes zoster and progression toward PHN. But, the results of our study revealed that Treg cell quantities were decreased compared to those in the control, although it was not statistically significant. Our findings conflict with the results of Xing et al.
3 and suggest that antiviral immune response increased in response to a reduction in Treg cells, leading to the decrease of VZV after outbreak of the disease, furthermore inflammatory responses increased via an increase in Th17 cells. We think there may be a difference between authors in herpes zoster. Perhaps, this may be discrepancy of blood sampling time in accordance with disease activity. For example, blood sample taken before outbreak of herpes zoster showed higher Treg cells count than herpes zoster negative control
17. In addition, these observations may be explained by the plasticity of the two T cell subsets, in which Treg cells are converted to Th17 cells by inflammatory cytokines owing to increased inflammatory responses, although this is controversial recently
1819. When analyzing the imbalance between the two T cell subsets or each T cell subset and the development of herpes zoster, the ratio of Th17 cells to Treg cells was significantly elevated in patients with herpes zoster compared with normal controls. The results of the present study confirm that the balance between Treg cells and Th17 cells is critical for the development of herpes zoster, similar to observations that an imbalance between Treg cells and Th17 cells, rather than each T cell subset alone, in chronic viral diseases, such as chronic HBV and HCV infections, affects progression and treatment
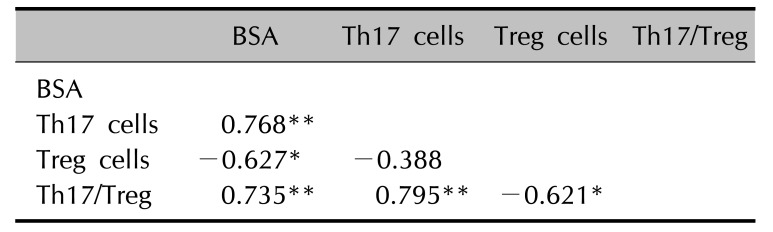
67. The analysis of herpes zoster-affected BSA, Treg cells, Th17 cells, and Th17/Treg ratios showed that significant positive correlations between BSA and Th17 cells and the Th17/Treg ratio and a significant negative correlation between BSA and Treg cells. Accordingly, the affected area may reflect cellular changes in the peripheral blood. So, we thought that Th17 cells may be associated with herpes zoster per se but also with the extent of lesions.
This study had several limitations. The number of participants was relatively small, and the onset time of herpes zoster differed among patients at the time of blood collection; this may explain differences in cellular changes. Treg cells generally tend to increase with age. Although the mean age of controls was higher than that of patients with herpes zoster, age-related changes were not considered in this study. In addition, when the proportions of Treg cells and Th17 cells in the peripheral blood were compared before and after treatment, blood was collected during a short period of time, i.e., 1 week, to observe changes in each cell type, based on the fact that that viral replication is generally regulated within 1∼2 weeks
9.
In the present study, the imbalance between Treg and Th17 cells and the development of herpes zoster was examined to determine the effects of Treg and Th17 cells on the reactivation of VZV and to examine the imbalance in Treg and TH17 cells and the development of herpes zoster.
In conclusion, the number of Th17 cells and the ratio of Th17 to Treg cells were significantly higher in patients with herpes zoster than in healthy controls. And, there was a significant correlation between the proportion and ratio of Treg and Th17 cells and the affected BSA. Therefore, Th17 was involved in herpes zoster and an imbalance between Treg cells and Th17 cells was observed in patients with herpes zoster. But, additional studies are needed to examine the mechanism by which Treg and Th17 cells influence the development of herpes zoster.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download