Abstract
Background
Human mannose-binding lectin (MBL) is a serum lectin taking part in the innate immunity by opsonizing various microorganisms for phagocytosis. The MBL serum concentration is affected by several single-nucleotide polymorphisms (SNPs) in the promoter region of the MBL2 gene.
Objective
The purpose of this study was to examine the relationship between MBL2 polymorphisms and atopic dermatitis (AD) susceptibility.
Methods
To examine whether the MBL2 SNPs are related to AD susceptibility, we examined 237 patients with AD and 94 controls by polymerase chain reaction (PCR)-restriction fragment length polymorphism and PCR-sequence specific primer analyses of four polymorphic loci: two (H/L and X/Y) within the promoter region and the other two (P/Q and A/B) within exon 1. MBL concentrations in the blood were estimated by ELISA.
Results
The prevalence of haplotype HYPB, leading to MBL deficiency, was significantly decreased in the AD patients compared to the controls (p=0.002), while the prevalence of haplotype HYPA was increased with a clear trend toward significance (p=0.056). The frequency of MBL2 LYPB/LXPA (odds ratio, 0.08; 95% confidence interval, 0.009~0.655; p=0.021) were significantly decreased in the AD patients. The blood log [total immunoglobulin E, IgE] levels of MBL2 HYPA/HYPA, HYPA/LYPA, HYPA/LYPB, HYPA/LYQA, and LYQA/LXPA haplotype pairs were significantly increased in the AD patients.
Conclusion
The frequency of MBL2 HYPB haplotype was significantly decreased in the AD patients compared to the controls. The frequency of LYPB/LXPA had a possibly protective effect on AD. Moreover, the MBL2 HYPA haplotype pairs, which were related to higher blood total IgE levels, were possibly associated with extrinsic AD.
Atopic dermatitis (AD) is a type of skin inflammation that is caused by both genetic and environmental factors1.
Human mannose-binding lectin (MBL) is a serum lectin taking part in the innate immunity by binding various microorganisms and activating the lectin-complement23. Opsonization defect due to deficiency of MBL is associated with increased susceptibility to infection and stems from the presence of a low efficiency promoter and/or three gene mutations in exon 1 (variants B, C, and D) of MBL223. The patients with abnormal homozygous MBL alleles are susceptible to an immune-deficiency disease that is not related to HIV4.
MBL deficiency could trigger AD or complications when they are additionally exposed to an infection. The first evidence of MBL involvement in AD was suggested by a family study which showed that children with low plasma MBL levels who were homozygous for allele B of MBL were prone to pruritic skin disease and possibly AD, with or without recurring infections5. In addition, a report revealed that low MBL levels were clearly associated with the BB MBL2 haplotype in a Turkish family members who also had recurrent skin infections and AD5. Moreover, the three exon 1 variants B, C, and D of MBL were more frequently observed in Brazilian AD patients than in healthy controls, although the disease severity was not investigated6. In this study, children with AD had higher frequency of allele O of MBL gene related to low or deficient levels of MBL compared to the control group6. Likewise, low or deficient serum MBL levels may result in predisposition to AD7, although there was a conflicting report that showed no association of the B allele of MBL with AD susceptibility in a Japanese population8.
We hypothesized that the modulation of innate immune defense by MBL2 variants influenced a wide range of susceptibility to AD. Therefore, we investigated whether single-nucleotide polymorphisms (SNPs) of the MBL2 gene could be proper genetic diagnostic factors in Korean AD patients by examining the SNPs and haplotypes, including −550 and −221 in the promoter region, +4 in the 5′ UTR and codon 54 in exon 1 of the MBL2 gene.
In this study, we included 237 unrelated Korean AD patients (132 males and 105 females; mean age 32.5±18.0 years; age range 5~90 years) who were registered in the Department of Dermatology, Uijeongbu St. Mary's Hospital in Korea. All patients showed moderate to severe AD according to the Hanifin's criteria9. Controls were 94 healthy persons without a personal or family history of AD. For genetic studies, blood was collected by venipuncture, and genomic DNA was prepared using QIAamp blood kit (QIAGEN, Hilden, Germany). Blood [total immunoglobulin E, IgE] was measured by LPIA-200 system (Iatron Corp., Tokyo, Japan). Blood IgE levels were in the range of 2~50,000 IU/ml (median [25th percentiles~75th percentiles], 282.5 [87.1~954.0]). Assays for specific IgE antibodies to Dermatophagoides pteronyssinus (Dp) and Dermatophagoides farina (Df) were performed with the Pharmacia CAP FEIA immunoassay on a UniCAP 100 automatic analyzer (Pharmacia and Upjohn, Uppsala, Sweden) according to the manufacturer's directions. An antigen-specific IgE value of over 0.35 kU/L was classified as increased. The clinical data are presented in Table 1. This study was carried out from March 3, 2003 to December 25, 2004 in compliance with the principles of the Declaration of Helsinki. Since there was no statutory law during that time, only verbal consent was obtained from the patients and healthy persons after explaining the purpose of our study and their rights. In case of children were included in the study, the parent or guardian was informed orally and they agreed to the purpose and procedure of our study. The coded research data obtained from March 3, 2003 to December 25, 2004 were reanalyzed and the Institutional Review Board (IRB) of Uijeongbu St. Mary's Hospital approved this study on November 12, 2015 (IRB no. UC15RISI0160).
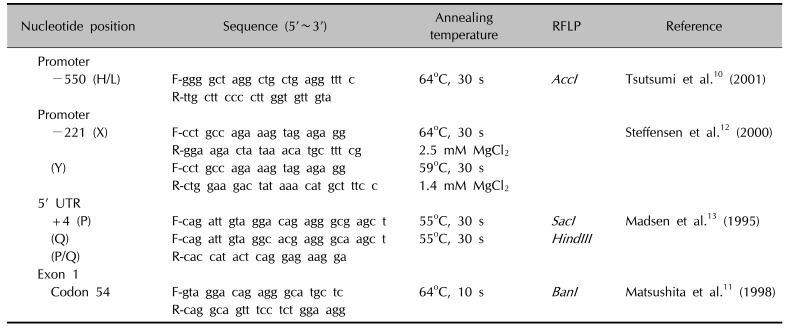
Primer sets were designed for the four polymorphic sites: H/L at −550, X/Y at −221, P/Q at +4, and A/B at codon 54 (Fig. 1A), and used for differential PCR analysis. Genotyping of the promoter region at position −550 (rs11003125) of the MBL2 gene and codon 54 (rs1800450) of exon 1 was carried out by the polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) analysis as described before1011. Genotyping of the promoter region at position −221 (rs7096206) of the MBL2 gene was performed by the PCR-sequence specific primer method, as described by Steffensen et al.12. DNAs with one point mutation in the 5′ UTR at position +4 (P/Q variants) of the MBL2 gene were amplified by site-directed mutagenesis (SDM)-PCR13. The P and Q alleles were detected by RFLP performed on the SDM-PCR products using a mutated 5′-primer (Table 2)10111213. AccI cleaved the 261 bp PCR product specific for the L allele into two fragments (239 bp and 22 bp), while BanI cleaved the A and B alleles. SacI and HindIII cleaved the 136 bp PCR products specific for the P and Q alleles into 110 bp and 26 bp fragments, respectively. PCR restriction fragments were separated by 8% polyacrylamide gel electrophoresis.
Hardy-Weinberg equilibrium was analyzed for the gene frequencies obtained by simple gene counting and the chi-square test was performed for comparing the observed and expected values. We examined a widely used measure of linkage disequilibrium (LD) between all pairs of bi-allelic loci, Lewontin's D′ (|D′|)15. Haplotype frequencies were calculated using the Phase 2.0 program, as described elsewhere16. Phase probabilities of each site were also calculated for each individual by the Phase 2.0 program. Individuals with phase probabilities less than 0.987 were excluded from the analysis. Genetic effects of inferred haplotypes were analyzed in the same way as SNPs. Comparison of genotype and haplotype frequencies for each MBL2 polymorphism was carried out by chi-square test. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using SAS ver. 8.1 (SAS Institute, Cary, NC, USA). p<0.05 after Bonferroni's adjustment for multiple testing of the four SNPs in the diplotype was considered to be statistically significant throughout the study. An OR provides an effect estimate, whereas a score of <1 is related to a protective effect, and a score of >1 is related to an increased risk. Genotype distribution of MBL2 SNPs and haplotypes among AD patients and normal controls were analyzed with logistic regression models adjusted for age, sex and log [total blood IgE] levels as covariates.
The clinical characteristics of 331 subjects are shown in Table 1. Mean age was higher in the controls compared to the patients, and males were predominated in both groups. Around 54% of the patients with AD were positive for Dp-specific IgE and 55% of patients were positive for Df-specific IgE. Subjects with AD had higher blood log [total IgE] levels (Student's t-test, p<0.0001).
The MBL2 gene located on chromosome 10q11.2-q21 consists of four exons with a total size of ~10.0 kb. Four SNPs in the MBL2 gene (Fig. 1A); three within the upstream of the promoter region (a G-C transversion at position 550, a C-G transversion at position 221, and a C-T transition at position +4 in the 5′ UTR) and one G-A transition at +230 calculated on the codon 54 of exon 1 were genotyped by the single-base extension method. As shown in Fig. 1, MBL −550 G>C, −221 G>C, +4 C>T and +230 G>A were included in the statistical analysis. All four SNPs were in complete (|D′|=1 and r2≠1) or absolute LD (|D′|=1 and r2=1). Seven haplotypes were identified without any ambiguous phasing due to LDs among SNPs (Fig. 1B, C).
Allele distributions of the polymorphisms on MBL2 (MBL −550 G>C, −221 G>C, +4 C>T, and +230 G>A) were determined in the study population (Table 3). No significant difference was found in the MBL2 polymorphism frequencies between AD patients and controls.
When haplotypes with a frequency greater than 0.01 were analyzed among the AD patients and controls, 7 haplotypes were observed at positions −550 and −221 in the promoter region, at +4 in the 5′ UTR, and at codon 54 in exon 1 of the MBL2. The frequency of the MBL2 HYPB haplotype was significantly decreased (p=0.002) in the AD patients (Table 4). The frequencies of the heterozygous genotype MBL2 LYPB/LXPA (OR, 0.08; 95% CI, 0.009~0.655; p=0.021) were also significantly decreased in the AD patients (Table 5).
The blood MBL levels were not significantly different between the AD patients and controls (mean±standard deviation: 3.30±2.40 vs. 3.06±2.05, respectively, p=0.077, Student's t-test). Interestingly, the blood MBL level was not detected in both AD patients and controls with the MBL2 HYPB/HYPB and HYPB/LYPB haplotypes, possibly leading to deficiency of MBL (Table 6).
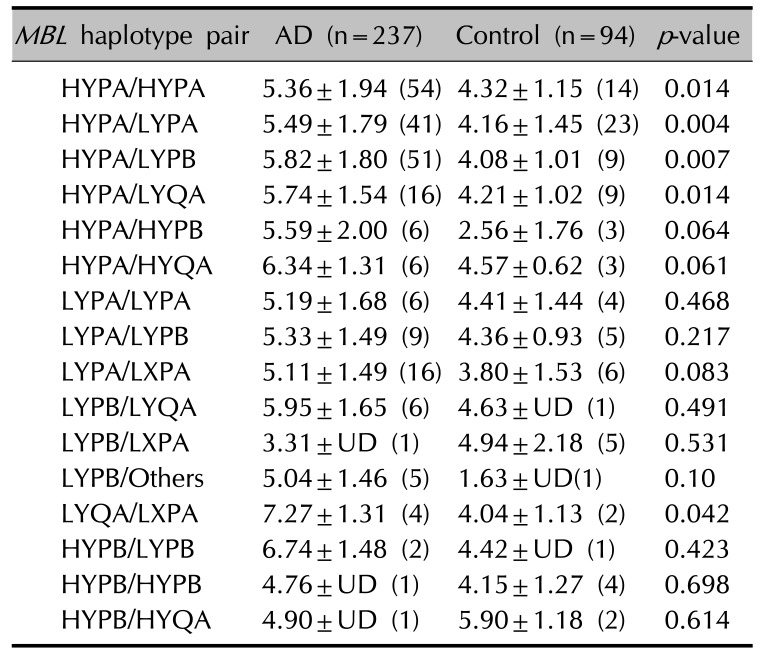
The blood MBL levels were significantly correlated with total IgE levels in the AD patients (r=0.13, p=0.048, Pearson's correlation). In addition, the blood log [total IgE] of MBL2 HYPA/HYPA, HYPA/LYPA, HYPA/LYPB, HYPA/LYQA, and LYQA/LXPA haplotype pairs were significantly increased in the AD patients compared to controls (Table 7).
In this study, we found a significant decrease in the frequency of MBL2 haplotype HYPB, −550G (H), −221G (Y), +4C (P), and codon 54Asp (B) in Korean AD patients. We also found that blood log [total IgE] levels of MBL2 HYPA/HYPA, HYPA/LYPA, HYPA/LYPB, HYPA/LYQA, LYQA/LXPA haplotype pairs were significantly increased in the AD patients.
In a previous study, higher frequency of point mutation at codon 54 (B) of the MBL gene has been implicated as a candidate susceptibility loci in Brazilian AD patients 7. We found that the frequency of MBL HYPB/HYPB haplotype was decreased in the AD patients, although the plasma MBL was not detected in both AD patients and controls with this haplotype and the number of subjects was too small for the statistical analysis (Table 6). MBL deficiency would not be a susceptibility factor for the development of certain complications. For example, it was demonstrated that AD patients with Eczema herpeticum (EH), a systemic complication caused by herpes simplex virus infection had similar levels and functional activities of serum MBL as AD patients with no history of EH17. Considering the sample size limitations in this study and our study, further research is needed to determine the detailed defects that predispose subjects to viral infection and AD.
Previous studies have demonstrated that there are large differences in the frequencies of exon 1 variant and haplotypes of MBL2 in various ethnic groups. For example, the codon 54 (B) mutant was found with a higher frequency in in Caucasians (0.22~0.28) and in Asians including Japanese and Korean patients (0.11~0.26), but very rare in Africans (0.0~0.03)11121819. In addition, the HYPA haplotype, the most effective MBL-producing haplotype, was found in Asians including Koreans (0.44~0.47) and Caucasians (0.25~0.34) and commonly in Eskimos (0.81). In contrast, the LXPA haplotype was found rarely in Asians including Koreans (0.07~0.10) and Eskimos (0.03), but it was found more commonly in Caucasians (0.18~0.26)3111218.
Various genetic polymorphisms in the MBL gene have been reported as risk factors for the development of a certain clinical subtype and severity. The susceptibility to Behcet's disease (BD) is related to a higher frequency of the MBL2 HYPA haplotype, which may cause increased acute or chronic hyper-inflammatory responses and influence the severity of BD19. A significantly higher percentage of patients with BD showed high serum MBL levels (≥500 ng/ml) compared to controls and were associated with skin lesions in Korean patients20. In addition, the progression of systemic lupus erythematosus is associated with MBL gene polymorphism and serum MBL concentration21.
In our study, the blood MBL levels were significantly correlated with total IgE levels in the AD patients. In addition, blood log [total IgE] levels of MBL2 HYPA/HYPA, HYPA/LYPA, HYPA/LYPB, HYPA/LYQA, and LYQA/LXPA haplotype pairs were significantly increased in the AD patients. Extrinsic AD shows high total plasma IgE levels and has specific IgE for environmental and food allergens, whereas intrinsic AD shows normal total IgE levels and the absence of specific IgE22. Our previous study demonstrated that the polymorphisms of the macrophage migration inhibitory factor (MIF) promoter related to innate immunity were significantly associated with an increased risk for AD. Especially, the −794 7-CATT locus and the MIF C/7-CATT haplotype were significantly associated with decrease of total IgE levels in the blood, suggesting that these polymorphisms might be a marker for intrinsic AD23. Therefore, the HYPA haplotype pair of the MBL2 gene, which is related to higher total blood IgE levels, would be a possible marker for extrinsic AD.
In conclusion, we investigated the SNPs and the haplotypes of the MBL2 gene, which might be proper genetic diagnostic factors in Korean AD patients. The frequency of MBL2 LYPB/LXPA had a possibly protective effect in Korean AD patients.
ACKNOWLEDGMENT
We thank Professor Kyung-Sook Park and M.S. Jin-Sun Choi, Department of Biology, Sungshin Women's University, Seoul, Korea for their helpful comments on the earlier version of our manuscript. This work was supported by a grant from the Korea Health 21 R&D Project, funded by the Ministry of Health and Welfare of Korea (01-PJ3-PG6-01GN09-003).
References
1. Akdis CA, Akdis M, Simon D, Dibbert B, Weber M, Gratzl S, et al. T cells and T cell-derived cytokines as pathogenic factors in the nonallergic form of atopic dermatitis. J Invest Dermatol. 1999; 113:628–634. PMID: 10504452.

2. Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992; 176:1497–1502. PMID: 1460414.

3. Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998; 161:3169–3175. PMID: 9743385.
4. Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 1995; 346:941–943. PMID: 7564730.

5. Brandrup F, Homburg KM, Wang P, Garred P, Madsen HO. Mannan-binding lectin deficiency associated with recurrent cutaneous abscesses, prurigo and possibly atopic dermatitis. A family study. Br J Dermatol. 1999; 140:180–181. PMID: 10215800.

6. Brandão LA, Guimarães RL, Carrera M, Milanese M, Segat L, Luiz de Lima-Filho J, et al. MBL2 functional allelic variants and increased risk for the development of atopic dermatitis in Brazilian children. Arch Dermatol. 2008; 144:412–413. PMID: 18347304.

7. Carréra MC, Moura P, Crovella S, de Souza PR, de Souza LC, Sarinho E. High polymorphism of the MBL2 gene in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2010; 105:39–42. PMID: 20642202.

8. Hashimoto S, Nakamura K, Oyama N, Kaneko F, Fujita T, Tsunemi Y, et al. Mannose-binding lectin (MBL) single nucleotide polymorphism is not associated with atopic dermatitis in Japanese patients. J Dermatol. 2005; 32:1038–1040. PMID: 16471473.

10. Tsutsumi A, Sasaki K, Wakamiya N, Ichikawa K, Atsumi T, Ohtani K, et al. Mannose-binding lectin gene: polymorphisms in Japanese patients with systemic lupus erythematosus, rheumatoid arthritis and Sjögren's syndrome. Genes Immun. 2001; 2:99–104. PMID: 11393663.

11. Matsushita M, Hijikata M, Ohta Y, Iwata K, Matsumoto M, Nakao K, et al. Hepatitis C virus infection and mutations of mannose-binding lectin gene MBL. Arch Virol. 1998; 143:645–651. PMID: 9638138.

12. Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Methods. 2000; 241:33–42. PMID: 10915847.

13. Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995; 155:3013–3020. PMID: 7673719.
14. Garred P, Madsen HO, Kurtzhals JA, Lamm LU, Thiel S, Hey AS, et al. Diallelic polymorphism may explain variations of the blood concentration of mannan-binding protein in Eskimos, but not in black Africans. Eur J Immunogenet. 1992; 19:403–412. PMID: 1477092.

15. Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987; 117:331–341. PMID: 3666445.

16. Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003; 73:1162–1169. PMID: 14574645.

17. Bundy KW, McGirt LY, Bankova LG, Wollenberg A, Beck LA, De Benedetto A. Mannan-binding lectin levels and activity are not altered in atopic dermatitis patients with a history of eczema herpeticum. Dermatol Res Pract. 2011; 2011:769890. PMID: 22110485.

18. Werth VP, Berlin JA, Callen JP, Mick R, Sullivan KE. Mannose binding lectin (MBL) polymorphisms associated with low MBL production in patients with dermatomyositis. J Invest Dermatol. 2002; 119:1394–1399. PMID: 12485445.
19. Park KS, Min K, Nam JH, Bang D, Lee ES, Lee S. Association of HYPA haplotype in the mannose-binding lectin gene-2 with Behçet's disease. Tissue Antigens. 2005; 65:260–265. PMID: 15730518.
20. Kim J, Im CH, Kang EH, Lee EY, Lee YJ, Park KS, et al. Mannose-binding lectin gene-2 polymorphisms and serum mannose-binding lectin levels in Behçet's disease. Clin Exp Rheumatol. 2009; 27:S13–S17. PMID: 19796526.
21. Takahashi R, Tsutsumi A, Ohtani K, Muraki Y, Goto D, Matsumoto I, et al. Association of mannose binding lectin (MBL) gene polymorphism and serum MBL concentration with characteristics and progression of systemic lupus erythematosus. Ann Rheum Dis. 2005; 64:311–314. PMID: 15647440.

22. Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010; 58:1–7. PMID: 20207111.

23. Kim JS, Choi J, Hahn HJ, Lee YB, Yu DS, Kim JW. Association of macrophage migration inhibitory factor polymorphisms with total plasma IgE Levels in patients with atopic dermatitis in Korea. PLoS One. 2016; 11:e0162477. PMID: 27598332.

Fig. 1
Gene map, haplotypes and linkage disequilibrium (LD) coefficients in MBL. (A) Gene map and single-nucleotide polymorphisms (SNPs) in MBL located on chromosome 10q11.2-q21. Polymorphic sites within the promoter region and exon-1 are marked by a gray block. Pairwise measures of linkage disequilibrium for the 4 polymorphisms are shown. (B) Haplotypes of MBL. Haplotypes with frequency >0.02 are presented. (C) LD coefficient (|D′|) among MBL SNP.
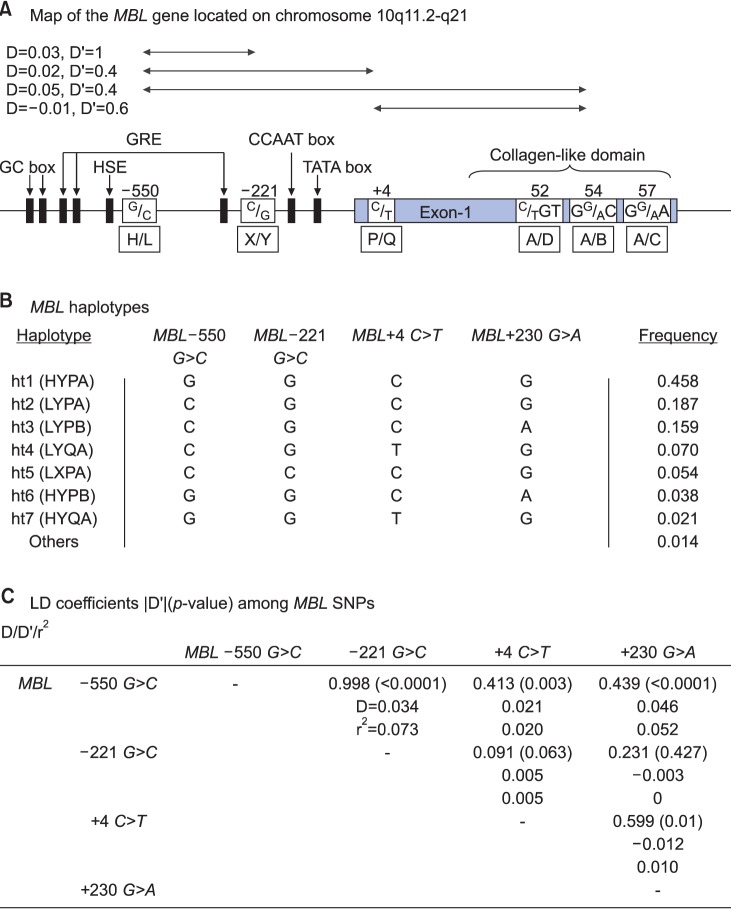
Table 1
Clinical profiles of the study subjects
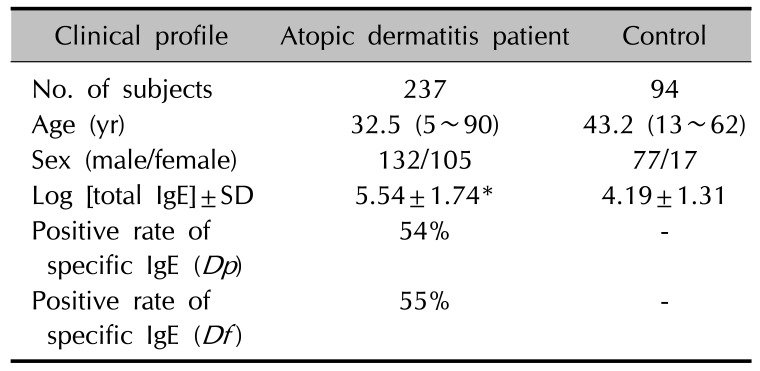
Table 2
Primers for MBL genotyping by PCR-RFLP, PCR-SSP and SDM-PCR RFLP
| Nucleotide position | Sequence (5'~3') | Annealing temperature | RFLP | Reference |
|---|---|---|---|---|
| Promoter | ||||
| −550 (H/L) | F-ggg gct agg ctg ctg agg ttt c | 64℃, 30 s | AccI | Tsutsumi et al.10 (2001) |
| R-ttg ctt ccc ctt ggt gtt gta | ||||
| Promoter | ||||
| −221 (X) | F-cct gcc aga aag tag aga gg | 64℃, 30 s | Steffensen et al.12 (2000) | |
| R-gga aga cta taa aca tgc ttt cg | 2.5 mM MgCl2 | |||
| (Y) | F-cct gcc aga aag tag aga gg | 59℃, 30 s | ||
| R-ctg gaa gac tat aaa cat gct ttc c | 1.4 mM MgCl2 | |||
| 5' UTR | ||||
| +4 (P) | F-cag att gta gga cag agg gcg agc t | 55℃, 30 s | SacI | Madsen et al.13 (1995) |
| (Q) | F-cag att gta ggc acg agg gca agc t | 55℃, 30 s | HindIII | |
| (P/Q) | R-cac cat act cag gag aag ga | |||
| Exon 1 | ||||
| Codon 54 | F-gta gga cag agg gca tgc tc | 64℃, 10 s | BanI | Matsushita et al.11 (1998) |
| R-cag gca gtt tcc tct gga agg |
Table 3
Genotype distribution of MBL polymorphisms in atopic dermatitis (AD) patients and normal controls (NC) in a Korean population
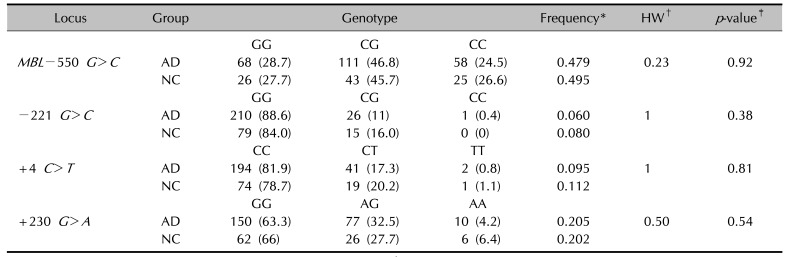
Table 4
Comparison of haplotype frequencies of the MBL gene between patients with AD and controls
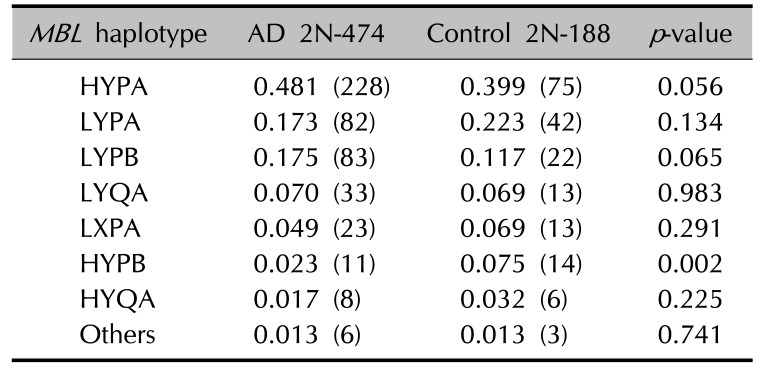
Table 5
Comparison of frequencies of MBL haplotype pairs between patients with AD and controls
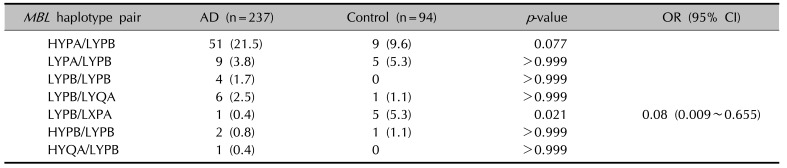
Table 6
Comparison of plasma MBL levels in MBL haplotype pairs between patients with AD and controls
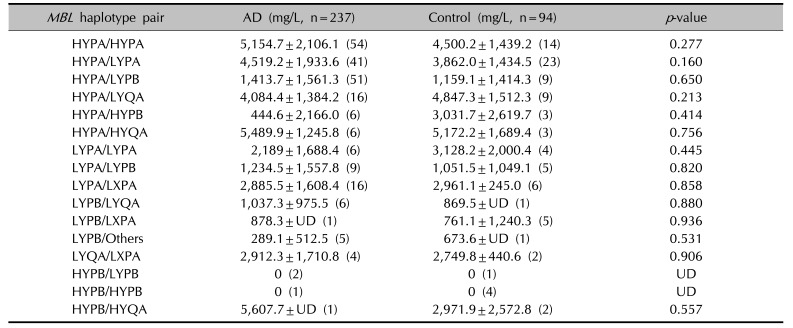
Table 7
Comparison of log [total immunoglobulin E] levels in MBL haplotype pairs between patients with AD and controls




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download