This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To report the radiological results of a pilot study for the Korean Lung Cancer Screening project conducted to evaluate the feasibility of lung cancer screening using low-dose chest computed tomography (LDCT) in Korea.
Materials and Methods
The National Cancer Center and three regional cancer centers participated in this study. Asymptomatic current or ex-smokers aged 55–74 years with a smoking history of at least 30 pack-years who had used tobacco within the last 15 years were considered eligible. In total, 256 participants underwent LDCT November 2016 through March 2017. The American College of Radiology Lung Imaging Reporting and Data System (Lung-RADS) was used to categorize the LDCT findings.
Results
In total, 57%, 35.5%, 3.9%, and 3.5% participants belonged to Lung-RADS categories 1, 2, 3, and 4, respectively. Accordingly, 7.4% participants exhibited positive findings (category 3 or 4). Lung cancer was diagnosed in one participant (stage IA, small cell lung cancer). Other LDCT findings included pulmonary emphysema (32.8%), coronary artery calcification (30.9%), old pulmonary tuberculosis (11.7%), bronchiectasis (12.9%), interstitial lung disease with a usual interstitial pneumonia pattern (1.2%), and pleural effusion (0.8%).
Conclusion
Even though the size of our study population was small, the positive rate of 7.4% was like or lower than those in other lung cancer screening studies. Early lung cancer was detected using LDCT screening in one participant. Lung-RADS may be applicable to participants in Korea, where pulmonary tuberculosis is endemic.
Go to :

Keywords: Lung cancer, Screening, Low dose, Computed tomography, Lung-RADS
INTRODUCTION
Lung cancer is the most common cause of cancer-related deaths in developing and developed countries. In Korea, lung cancer has been the leading cause of cancer-related death since 1999, accounting for 22.8% of all cancer-related deaths in 2014 (
1). The 5-year relative survival rate for localized lung cancer is 46.3%, while that for lung cancer with distant metastasis is only 4.8% (
2). Therefore, early detection of lung cancer has a significant impact on lung cancer surveillance.
In the National Lung Screening Trial (NLST), a randomized clinical trial, low-dose chest computed tomography (LDCT) screening resulted in 20% decrease in lung cancer-specific mortality and 6.7% decrease in the overall mortality in high-risk patients compared with conventional chest radiography (
3). In 2015, a Korean multi-society collaborative committee announced guidelines for lung cancer screening, that also recommended annual LDCT screening for current smokers and ex-smokers (if less than 15 years have elapsed after smoking cessation) aged 55–74 years with a smoking history of 30 pack-years or more (
4).
From these perspectives, the Korean Lung Cancer Screening (K-LUCAS) project was launched. Prior to the K-LUCAS project, a pilot study was conducted to evaluate the feasibility of lung cancer screening protocol using LDCT in Korea by piloting the standardized protocols for selection of subjects, LDCT scan and reading by implementing Lung Imaging Reporting and Data System (Lung-RADS). Here we report the radiological results of this pilot study.
Go to :

MATERIALS AND METHODS
Participants
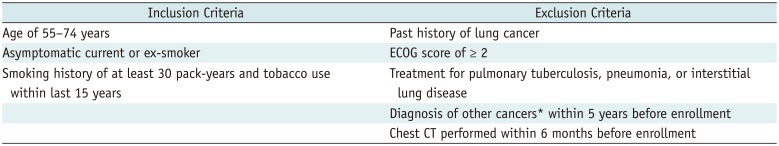
The National Cancer Center and three regional cancer centers of Korea (Busan Regional Cancer Center, Chungbuk Regional Cancer Center, Incheon Regional Cancer Center) participated in this pilot study, approved by the Institutional Review Board of each participating center. Our goal was to enroll 400 participants (100 per each center) November 2016 through March 2017, either from health check-up centers used for the National Health Screening Program or National Cancer Screening, or from smoking cessation clinics. Informed consent was obtained from all participants. They were assessed for screening eligibility by medical coordinators using a questionnaire including questions pertaining to the smoking history and health condition. Based on Korean guidelines for lung cancer screening (
4), we considered asymptomatic current or ex-smokers aged 55–74 years with a smoking history of at least 30 pack-years who had used tobacco within the last 15 years eligible for participation. Subjects with a history of lung cancer, those requiring assistance in daily life or ambulation (Eastern Cooperative Oncology Group score ≥ 2), and those receiving treatment for pulmonary tuberculosis, pneumonia, or interstitial lung disease were excluded. Subjects with a diagnosis of cancer (except thyroid cancer and skin cancer) within the last 5 years and those that had undergone chest computed tomography (CT) within the last 6 months were also excluded (
Table 1).
Table 1
Inclusion and Exclusion Criteria for Our Pilot Study for K-LUCAS Project
|
Inclusion Criteria |
Exclusion Criteria |
|
Age of 55–74 years |
Past history of lung cancer |
|
Asymptomatic current or ex-smoker |
ECOG score of ≥ 2 |
|
Smoking history of at least 30 pack-years and tobacco use within last 15 years |
Treatment for pulmonary tuberculosis, pneumonia, or interstitial lung disease |
|
Diagnosis of other cancers* within 5 years before enrollment |
|
Chest CT performed within 6 months before enrollment |

LDCT
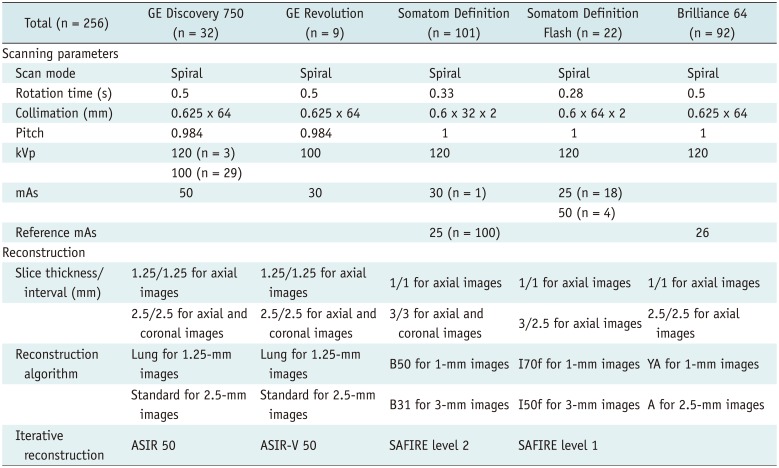
Considering that each center used various CT scanners and imaging protocols, we enforced minimal requirements for CT scanners and standardization of acquisition parameters as follows: multidetector CT with a minimum of 16 channels, complete scan with one-breath hold, thick (≤ 3 mm)- and thin (≤ 1.25 mm)-slice axial images, gantry rotation time of < 0.5 seconds, and a volume CT dose index (CTDIvol) of < 3.0 mGy for average-size-persons (170 cm/70 kg; body mass index [BMI], 24 kg/m
2). Additional coronal or sagittal reconstruction images were recommended, and techniques such as automatic exposure control or iterative reconstruction were also recommended to minimize the radiation dose. Detailed LDCT scanning and reconstruction protocols used in this pilot study are described in
Table 2.
Table 2
LDCT Scanners and Scanning Parameters Used in Our Pilot Study for K-LUCAS Project
|
Total (n = 256) |
GE Discovery 750 (n = 32) |
GE Revolution (n = 9) |
Somatom Definition (n = 101) |
Somatom Definition Flash (n = 22) |
Brilliance 64 (n = 92) |
|
Scanning parameters |
|
|
|
|
|
|
Scan mode |
Spiral |
Spiral |
Spiral |
Spiral |
Spiral |
|
Rotation time (s) |
0.5 |
0.5 |
0.33 |
0.28 |
0.5 |
|
Collimation (mm) |
0.625 × 64 |
0.625 × 64 |
0.6 × 32 × 2 |
0.6 × 64 × 2 |
0.625 × 64 |
|
Pitch |
0.984 |
0.984 |
1 |
1 |
1 |
|
kVp |
120 (n = 3) |
100 |
120 |
120 |
120 |
|
100 (n = 29) |
|
|
|
|
|
mAs |
50 |
30 |
30 (n = 1) |
25 (n = 18) |
|
|
|
|
|
50 (n = 4) |
|
|
Reference mAs |
|
|
25 (n = 100) |
|
26 |
|
Reconstruction |
|
|
|
|
|
|
Slice thickness/interval (mm) |
1.25/1.25 for axial images |
1.25/1.25 for axial images |
1/1 for axial images |
1/1 for axial images |
1/1 for axial images |
|
2.5/2.5 for axial and coronal images |
2.5/2.5 for axial and coronal images |
3/3 for axial and coronal images |
|
2.5/2.5 for axial images |
|
Reconstruction algorithm |
Lung for 1.25-mm images |
Lung for 1.25-mm images |
B50 for 1-mm images |
I70f for 1-mm images |
YA for 1-mm images |
|
Standard for 2.5-mm images |
Standard for 2.5-mm images |
B31 for 3-mm images |
I50f for 3-mm images |
A for 2.5-mm images |
|
Iterative reconstruction |
ASIR 50 |
ASIR-V 50 |
SAFIRE level 2 |
SAFIRE level 1 |
|

Analysis of LDCT Findings
We used Lung-RADS proposed by the American College of Radiology to categorize participants according to the initial LDCT findings (
5). Lung-RADS categories 1 and 2 (negative and benign appearance, respectively) represent negative screening results, while categories 3 (probably benign) and 4 (suspicious) represent positive results. LDCT findings were interpreted by thoracic radiologists at each center using LungReport (Infinitt Healthcare, Seoul, Korea), developed for automated categorization of the findings into Lung-RADS categories based on the size and characteristics of nodules.
For comparison of the positive rate, we classified the nodules using the NLST criteria (any noncalcified nodules equal to or larger than 4 mm in any diameter) (
3). We assessed the rate of compliance with the recommendations in subjects with positive findings.
Radiation Dose
The CTDIvol and dose-length product (DLP) values were obtained from automatically generated patient protocols. The effective radiation dose during LDCT was calculated as the DLP multiplied by a region-specific conversion coefficient (0.014 mSv/mGy-cm) (
6).
Go to :

RESULTS
Participants
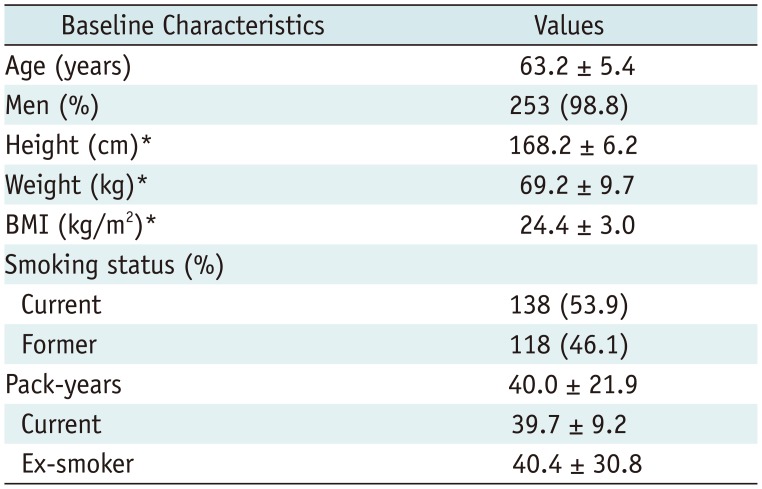
In total, 256 participants underwent LDCT (
Table 3). The mean age of participants was 63.2 ± 5.4, and 98.8% (n = 253) were men. In total, 138 subjects (53.9%) were current smokers with a mean smoking history of 39.7 ± 9.2 pack years and 118 (46.1%) were ex-smokers with a mean smoking history of 40.4 ± 30.8 pack years. This was the first LDCT screen for most participants (92.6%); 19 (7.4%) had undergone chest CT more than 6 months (61.1 ± 46.0 months; range, 7–168 months) before enrollment.
Table 3
Baseline Characteristics of Participants in Our Pilot Study for K-LUCAS Project
|
Baseline Characteristics |
Values |
|
Age (years) |
63.2 ± 5.4 |
|
Men (%) |
253 (98.8) |
|
Height (cm)*
|
168.2 ± 6.2 |
|
Weight (kg)*
|
69.2 ± 9.7 |
|
BMI (kg/m2)*
|
24.4 ± 3.0 |
|
Smoking status (%) |
|
|
Current |
138 (53.9) |
|
Former |
118 (46.1) |
|
Pack-years |
40.0 ± 21.9 |
|
Current |
39.7 ± 9.2 |
|
Ex-smoker |
40.4 ± 30.8 |

Findings of LDCT Screening
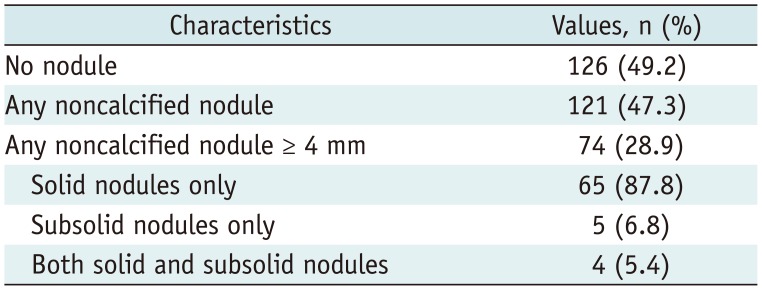
The distribution of nodules detected on LDCT screening and the Lung-RADS categories based on initial LDCT findings are shown in
Tables 4 and
5, respectively. In total, 121 participants (47.3%) exhibited non-calcified pulmonary nodules and 126 (49.2%) did not exhibit pulmonary nodules (
Table 4). Non-calcified nodules measuring ≥ 4 mm were detected in 74 participants (28.9%) (
Table 4). In total, 57.1% (n = 146), 35.5% (n = 91), 3.9% (n = 10), and 3.5% (n = 9) participants belonged to Lung-RADS categories 1, 2, 3, and 4, respectively. Four participants were classified under category 2 instead of category 3 (n = 3) or category 4A (n = 1) because the nodules remained unchanged relative to the findings on previous chest CT. Therefore, 7.4% (n = 19) participants had positive results (category 3 or 4) (
Table 5). The positive rate decreased by 21.5% when Lung-RADS was used instead of the NLST criteria. Of the 19 participants with positive results recommended for follow-up studies or additional study for evaluation of nodules, only 10 (52.6%) had completed follow-up studies; the average follow-up duration was 7 ± 1 months (range, 5–8 months). To be specific, two category 3 nodules and five category 4A nodules were unchanged and coded as category 2. One category 3 nodule and one category 4A nodule disappeared. Chest CT with contrast enhancement and following endobronchial ultrasound-guided transbronchial lung biopsy under fluoroscopic guidance was performed for one participant in category 4B; the final diagnosis was stage IA small cell lung cancer.
Table 4
Distribution of Nodules Detected on LDCT Screening in Our Pilot Study for K-LUCAS Project
|
Characteristics |
Values, n (%) |
|
No nodule |
126 (49.2) |
|
Any noncalcified nodule |
121 (47.3) |
|
Any noncalcified nodule ≥ 4 mm |
74 (28.9) |
|
Solid nodules only |
65 (87.8) |
|
Subsolid nodules only |
5 (6.8) |
|
Both solid and subsolid nodules |
4 (5.4) |

Table 5
American College of Radiology Lung-RADS Categories Based on Initial Findings of LDCT in Our Pilot Study for K-LUCAS Project
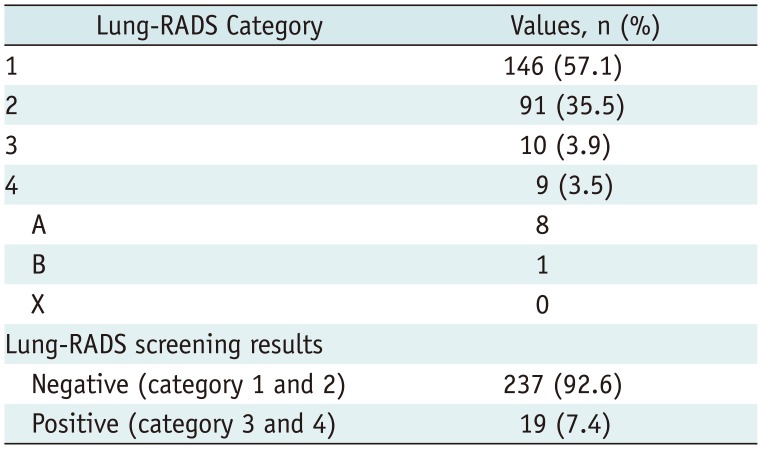
|
Lung-RADS Category |
Values, n (%) |
|
1 |
146 (57.1) |
|
2 |
91 (35.5) |
|
3 |
10 (3.9) |
|
4 |
9 (3.5) |
|
A |
8 |
|
B |
1 |
|
X |
0 |
|
Lung-RADS screening results |
|
|
Negative (category 1 and 2) |
237 (92.6) |
|
Positive (category 3 and 4) |
19 (7.4) |

Other CT findings included pulmonary emphysema (n = 84, 32.8%), coronary artery calcification (n = 79, 30.9%), old pulmonary tuberculosis (n = 30, 11.7%), bronchiectasis (n = 33, 12.9%), interstitial lung disease with a usual interstitial pneumonia pattern (n = 3, 1.2%), and pleural effusion (n = 2, 0.8%).
Radiation Dose
Table 6 shows the radiation dose used for LDCT. The height, weight, and radiation dose were not recorded for three participants. A total of 39 scans used 100 kVp instead of 120 kVp (
Table 2). The mean CTDIvol was 1.7 ± 0.6 mGy and the effective radiation dose was 1.0 ± 0.3 mSv. In 9 participants, CTDIvol exceeded 3 mGy because of overweight/obesity (BMI ≥ 25 kg/m
2, n = 7) and/or the use of a higher tube current setting of 50 mA (n = 4).
Table 6
Radiation Dose Used for LDCT in Our Pilot Study for K-LUCAS Project
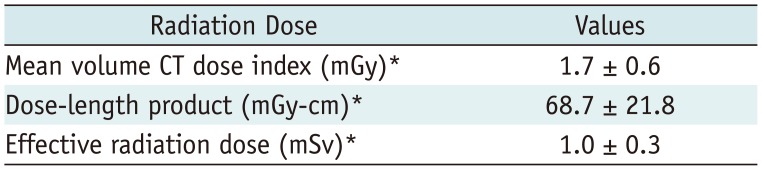
|
Radiation Dose |
Values |
|
Mean volume CT dose index (mGy)*
|
1.7 ± 0.6 |
|
Dose-length product (mGy-cm)*
|
68.7 ± 21.8 |
|
Effective radiation dose (mSv)*
|
1.0 ± 0.3 |

Go to :

DISCUSSION
This study documents the radiological findings of a pilot study for the K-LUCAS project conducted to develop a suitable protocol for lung cancer screening and overcome the limitations of the protocol in 2016. The K-LUCAS project was planned as a multicenter cohort study of 8000 participants and was initiated in 2017 for evaluation of the feasibility of nationwide lung cancer screening using LDCT in Korea. In the present pilot study, we targeted 400 participants; however, only 256 were enrolled. Of 256 participants, 7.4% exhibited positive results based on Lung-RADS. This rate was lower than the positivity rate (27.3%) based on the NLST criteria. Lung cancer was diagnosed in one (0.4%) participant after an initial round of screening.
To evaluate the feasibility of national lung cancer screening using LDCT, we must consider the high prevalence of tuberculosis in Korea. According to the 2015 World Health Organization database, a total of 40847 tuberculosis cases were recorded in Korea, where the incidence of tuberculosis of 80 per 100000 individuals is much higher than that in the U.S. (3.2 per 100000) (
7). Because of the high prevalence of tuberculosis in Korea, pulmonary nodules found on screening are much more in numbers compared with those found in Western populations (
8). In this pilot study, 11.7% participants were diagnosed with old pulmonary tuberculosis using LDCT. The prevalence of non-calcified pulmonary nodules (47.3%) was not much higher than that in previous studies including high-risk smokers (43%–76%) from Western countries (
91011). The rate of positive results (category 3 or 4, 7.4%) obtained based on Lung-RADS was lower than that (12.6%) in an NLST trial (
12). Although follow-up studies are necessary to exclude false-negative results associated with the use of Lung-RADS, we believe that Lung-RADS may be applicable to screening participants in Korea, where pulmonary tuberculosis is endemic.
Lung-RADS can standardize LDCT reporting and management recommendations, decrease confusion during LDCT interpretation, and decrease the false-positive rate in lung cancer screening (
512). However, considerable error in the classification of nodules into Lung-RADS categories by thoracic radiologists has been observed because of its complexity (
13). Therefore, we used software developed for automated categorization of LDCT findings into Lung-RADS categories. This software also generates the CT reading form automatically. With the use of this software, time was saved and errors in lung-RADS categorization were minimized. The K-LUCAS project, planned to be implemented 2017–2018, is using a more upgraded reading program with viewer and computer assisted diagnosis functions. The results of this multicenter cohort study will be reported in the near future.
In this study, only one primary lung cancer was identified among 256 participants. Thus, the lung cancer detection rate was 0.4%. The diagnosis was stage IA small cell carcinoma, and appropriate treatment was administered. Although the lung cancer detection rate in our study was lower than that in previously published studies (
39141516), we cannot deduce a conclusion because of the small number of participants in our study. However, our findings demonstrate that early lung cancer can be detected with LDCT screening, and that lung cancer screening is feasible in a community setting.
This study has several limitations. First, the sample size was relatively small. We targeted 400 individuals but enrolled 256 because of the short duration and lack of publicity. For the selection of appropriate candidates according to the accurate smoking history, we recruited participants only through health check-up centers or smoking cessation clinics, not through self-referral programs. Second, 9 of the 19 participants with positive results did not follow the management recommendations because they were given a free choice to follow the recommendations. Third, because of the short-term follow-up period, we could not analyze the false-positive rate. The incidence of lung cancer could have been underestimated. Therefore, additional follow-up studies are required for the clinical outcome data.
In conclusion, the rate of positive results based on Lung-RADS in our study was 7.4%, which, despite the small sample size, was similar to or lower than the rates in previous lung cancer screening studies. Early lung cancer was detected using LDCT screening in one participant. Lung-RADS may be applicable to screening participants in Korea, where pulmonary tuberculosis is endemic.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download