Abstract
Objective
To investigate the value of diffusion-weighted magnetic resonance imaging (DW-MRI) as a noninvasive tool to assess salivary gland function for follow-up of patients with radiation-induced xerostomia.
Materials and Methods
This study included 23 patients with nasopharyngeal carcinoma who had been treated with parotid-sparing radiotherapy (RT). Salivary function was assessed by DW-MRI pre-treatment and one week and one year post-RT, respectively. The maximum apparent diffusion coefficient (ADC) of parotid glands (pADCmax) and the time to peak ADC of parotid glands (pTmax) during stimulation were obtained. Multivariate analysis was used to analyze factors correlated with the severity of radiation-induced xerostomia.
Results
The ADCs of parotid and submandibular glands (1.26 ± 0.10 × 10−3 mm2/s and 1.32 ± 0.07 × 10−3 mm2/s pre-RT, respectively) both showed an increase in all patients at one week post-RT (1.75 ± 0.16 × 10−3 mm2/s, p < 0.001 and 1.70 ± 0.16 × 10−3 mm2/s, p < 0.001, respectively), followed by a decrease in parotid glands at one year post-RT(1.57 ± 0.15 × 10−3 mm2/s, p < 0.001) but not in submandibular glands (1.69 ± 0.18 × 10−3 mm2/s, p = 0.581). An improvement in xerostomia was found in 13 patients at one year post-RT. Multivariate analysis revealed 4 significant predictors for the improvement of xerostomia, including dose to parotid glands (p = 0.009, odds ratio [OR] = 0.639), the ADC of submandibular glands (p = 0.013, OR = 3.295), pADCmax (p = 0.024, OR = 0.474), and pTmax (p = 0.017, OR = 0.729) at one week post-RT.
Salivary glands are commonly irradiated with a high dose of radiotherapy (RT) when treating head and neck cancers, especially nasopharyngeal carcinoma (NPC) (1). Radiation damage to salivary glands and consequent hyposalivation can appear early in the course of treatment and can result in varying degrees of xerostomia (2), which may reduce the quality of life in long-term survivors and pose a new, distressing health problem for them. Although the risk of xerostomia has been reduced with the use of intensity modulated radiotherapy (IMRT), it is not always possible to spare major salivary glands, and xerostomia is still one of the most frequent complications resulting from RT (345).
Currently, there are a variety of different methods available for the assessment of radiation-induced xerostomia. Salivary flow rate measurement is the most commonly applied objective technique, but its low reproducibility may lead to an inconsistent result and limit the usefulness of this measurement modality (6). Salivary gland scintigraphy has also been widely used to evaluate salivary gland function. However, it involves the use of ionizing radiation, and its spatial resolution is unsatisfactory (7).
Diffusion-weighted magnetic resonance imaging (DW-MRI) is an imaging technique able to detect the Brownian motion of water molecules and to quantify the diffusion by measuring the apparent diffusion coefficient (ADC). It has shown its value in the assessment of normal salivary glands, salivary tumors, and non-neoplastic disorders affecting the salivary glands (891011). A few previous studies have already dealt with DW-MRI in irradiated salivary glands, but there are considerable discrepancies with respect to the changes in ADC after RT (12131415). Moreover, the correlation between ADC values and decreased salivary function remains unclear, and there are no existing reports concerning DW-MRI in a long-term follow-up of radiation-induced xerostomia.
The aim of this study was to investigate the value of DW-MRI with salivary secretion stimulation as a noninvasive tool to assess salivary gland function for follow-up of patients with radiation-induced xerostomia.
The local institutional ethics committee granted ethical approval for this study, and each patient provided informed written consent prior to participation.
From August 2009 to April 2013, 28 patients with biopsy-proven and previously untreated NPC were enrolled for this prospective study. Five patients were excluded on the basis of local recurrence (n = 2) and distant metastasis (n = 3) within one year after RT, resulting in a total of 23 patients (8 women and 15 men; median age, 46 years; age range, 35–59 years) included in the analysis.
All patients received 9-field IMRT with 6 MV photon beams and chemotherapy with docetaxel, cisplatin, and 5-fluorouracil. Dose prescription was 60–70 Gy to the macroscopic disease, 60 Gy to the regions at high risk for microscopic disease, and 54 Gy to the regions at low risk for microscopic disease, administered over 6.0–6.5 weeks with a daily fraction of 2.2 Gy. Treatment planning was performed on the Pinnacle3® treatment planning system (Philips Healthcare, Best, the Netherlands). Whenever possible, the mean doses to bilateral parotid glands were kept at < 26 Gy. Dose constraints were not given to the submandibular glands.
MRI was performed on each patient before the start of RT and 1 week and 1 year after the completion of RT. Clinical xerostomia was assessed at the same time that MRI was conducted, according to the Radiation Therapy Oncology Group (RTOG) radiation morbidity scoring criteria. None of the patients had a history of salivary gland disease before RT.
A 3T MR system (SignaHDx; GE Healthcare, Milwaukee, WI, USA) with a maximum gradient capability of 23 mT/m and an 8-channel neurovascular head and neck array coil was used to obtain MR images. All patients were asked to fast for at least 1 hour before the examination. Initially, a T1-weighted spin-echo series (echo time [TE] = 14 ms, repetition time [TR] = 900 ms, matrix size = 192 × 384, section thickness = 6 mm, intersection gap = 1.5 mm, field of view [FOV] = 180 × 240 mm) and a T2-weighted fast spin-echo series (TE = 126 ms, TR = 1600 ms, matrix size = 160 × 320, section thickness = 8 mm, intersection gap = 1 mm, FOV = 220 × 280 mm) were performed in the transvers plane for anatomical localization and morphologic evaluation of the salivary glands. The images encompassed the area from the skull base to the undersurface of the submandibular glands, including entire parotid and submandibular glands.
Thereafter, an axial echo-planar diffusion-weighted imaging (DWI) sequence was obtained with a TR of 6000 ms, a minimum TE of 80 ms, a matrix of 128 × 96, and 2 excitations. The other imaging parameters, including FOV, section thickness, and intersection gap, were identical to those for T2-weighted imaging. Five different b values (b = 0, 400, 600, 800, and 1000 s/mm2) were used. These motion-probing gradients were applied in each of three orthogonal directions with the same strength. The parotid and submandibular glands were scanned respectively, and two DWI sequences were acquired at rest. The acquisition time of each sequence was 2 minutes 42 seconds, and nine sections that covered entire parotid or submandibular glands were obtained.
Subsequently, six 100 mg tablets of commercially available ascorbic acid were given orally. Patients were instructed to let the tablets melt in their mouths without chewing. During the salivary stimulation, the DWI sequence was repeated 7 times on parotid glands, with intervals of 18 seconds between consecutive sequences and a total scan time of 21 minutes.
Apparent diffusion coefficient maps were automatically constructed for all DWI images by means of pixel-by-pixel calculation. The ADC values were calculated using the following equation: S(i) = S0 × exp (−bi × ADC), where S(i) is the signal intensity measured on the ith b value image, bi is the corresponding b value, and S0 is a variable indicating the exact signal intensity for b = 0 s/mm2. To reduce the influence of noise on the calculations, diffusion images with all the applied b factors were used.
The data were digitally transferred from the MRI unit console to an independent Linux workstation with dedicated software (Advantage Workstation Version 4.3; GE Healthcare). On the native DWI images, regions of interest (ROIs) were manually drawn on three contiguous slices on the midsection of each gland, including as much of the gland parenchyma as possible but excluding the regions containing large vessels such as the retromandibular vein and external carotid artery (Fig. 1). T2-weighted images were used to guide the delineation of the glands. These ROIs were automatically copied to the corresponding ADC maps on all sequences before and during the stimulation for ADC calculations. Measurements obtained from the three contiguous slices in each gland were averaged for further data analysis. The mean ADC of parotid glands at rest (pADC) and submandibular glands at rest (sADC), the maximum ADC of parotid glands (pADCmax), and the time to peak ADC of parotid glands (pTmax) after stimulation were obtained. For comparison, a circular ROI of 60 mm2 was also placed in the center of the pons for each subject before and after RT. All the measurements of ADC values were performed independently by two radiologists with 9 years' (Reader 1) and 30 years' (Reader 2) experience in head and neck imaging. To assess intra-observer reproducibility, Reader 1 repeated the measurements twice in a 2-week period and recorded the ADC values again.
The numerical data were presented as absolute numbers and mean ± standard deviation. Kolmogorov-Smirnov's test was used to determine whether the parameters from DWI were normally distributed. Paired 2-tailed Student's t tests were used to compare the doses and the ADC values of parotid and submandibular glands. To compare the DW-MRI findings at different times before and after RT, two-way analysis of variance and the Bonferroni correction were conducted. Clinical data including sex, age, radiation doses, and ADC-related parameters between patients with different degrees of xerostomia were compared using an unpaired Student's t test, a chi-square and a Wilcoxon test as appropriate. Multivariate stepwise logistic regression analysis with forward selection was conducted by considering the alleviation of xerostomia as a target variable and the best predictors from the univariate tests as explanatory variables. The variables with a p value < 0.15 were entered in the model. Calculations of an intra-class correlation coefficient (ICC) were used to determine the level of reproducibility for ADCs. A p value less than 0.05 was considered to be a statistically significant difference. All statistical analyses were performed with SPSS Statistics software (Version 20.0; IBM Corp., Armonk, NY, USA).
All 23 patients completed IMRT. Clinical xerostomia measured by RTOG criteria was Grade 0 before RT and Grade 2 at 1 week post-RT in all patients. At 1 year post-RT, xerostomia changed to Grade 1 in 56.5% (13/23) patients, and it was still Grade 2 in the remaining 43.5% (10/23) patients. There was no significant difference in sex and age between the two groups (p = 0.551 and 0.516, respectively). The mean doses to salivary glands are provided in Table 1. The dose to parotid glands in patients with Grade 1 xerostomia was significantly lower than that in patients with Grade 2 (p < 0.001), but there was no significant difference on submandibular glands between the two groups (p = 0.100).
Satisfactory reproducibility of the measurement of ADC values was achieved. The inter-observer ICC calculated on the basis of the measurements from two radiologists was 0.91 (p < 0.001), and the intra-observer ICC calculated based on the two measurements from Reader 1 was 0.94 (p < 0.001). Therefore, all outcomes were based on the first measurements from Reader 1.
The ADCs of salivary glands before and after RT were shown in Table 2. After acid stimulation, the ADC of parotid glands increased from 1.26 ± 0.10 × 10−3 mm2/s to 1.48 ± 0.18 × 10−3 mm2/s before RT (p < 0.001), from 1.75 ± 0.16 × 10−3 mm2/s to 2.03 ± 0.23 × 10−3 mm2/s at 1 week after RT (p < 0.001), and from 1.57 ± 0.15 × 10−3 mm2/s to 1.79 ± 0.14 × 10−3 mm2/s at 1 year after RT (p < 0.001). Compared with the pre-RT values, the pADC and the pADCmax both showed an increase at 1 week post-RT (p < 0.001), followed by a decrease at 1 year post-RT (p < 0.001), but still higher than the baseline values (Fig. 2). The sADC also showed an increase from 1.32 ± 0.07 × 10−3 mm2/s to 1.70 ± 0.16 × 10−3 mm2/s at 1 week post-RT (p < 0.001), but without a decrease at 1 year post-RT (1.69 ± 0.18 × 10−3 mm2/s, p = 0.581) (Fig. 2). The pTmax post-RT became longer than that before RT (p < 0.001). As a reference tissue, the ADCs of the pons showed no significant change during the entire investigation period.
Grouped by clinical xerostomia at 1 year post-RT, the DW-MRI findings are presented in Table 2. Before RT, the pADC (1.25 ± 0.10 × 10−3 mm2/s), pADCmax (1.47 ± 0.17 × 10−3 mm2/s), pTmax (9.92 ± 6.83 minutes), and sADC (1.32 ± 0.08 × 10−3 mm2/s) in patients with Grade 1 xerostomia showed no significant difference with those in patients with Grade 2 (1.27 ± 0.11 × 10−3 mm2/s, 1.49 ± 0.19 × 10−3 mm2/s, 10.20 ± 5.54 minutes, and 1.32 ± 0.07 × 10−3 mm2/s, respectively). At 1week post-RT, patients with Grade 1 xerostomia had a shorter pTmax (10.85 ± 7.00 minutes, p = 0.019) and a higher sADC (1.75 ± 0.12 × 10−3 mm2/s, p = 0.013) than those with Grade 2 (15.45 ± 5.36 minutes and 1.64 ± 0.19 × 10−3 mm2/s, respectively) (Figs. 3, 4, 5). At 1 year post-RT, the differences between the two groups disappeared.
The multivariate regression analysis revealed 4 significant predictors for the improvement of xerostomia, including dose to parotid glands (p = 0.009, odds ratio [OR] = 0.639), sADC (p = 0.013, OR = 3.295), pADCmax (p = 0.024, OR = 0.474), and pTmax (p = 0.017, OR = 0.729) at 1 week post-RT.
In this prospective study, a DWI protocol was used to evaluate the changes of major salivary glands in NPC patients before and after parotid-sparing IMRT. We confirmed previous findings and add additional data about ADC changes in irradiated salivary glands at different time points after RT (16). To our knowledge, the current study, with a long-term follow-up, is the first to demonstrate the difference between acute and late radiation-induced xerostomia using functional parameters defined by DWI. Meanwhile, our results revealed significant correlations between early ADC-related parameters and late xerostomia grades. One week after RT, patients with a lower parotid dose, lower pADCmax, shorter pTmax, and higher sACD were more likely to show improvement rather than a permanent loss of salivary gland function. As such, DWI-based prediction of xerostomia development could be used to identify patients who require more aggressive investigations for salivary function, therefore enabling earlier preventive or curative interventions.
Radiation-induced hyposalivation often occurs in the early stage of RT. A significant decrease in salivary secretion has been documented in the first days after irradiation (217). In this series, xerostomia measured by RTOG criteria changed from Grade 0 to Grade 2 in all patients at 1 week post-RT, suggesting acute reduction of salivary gland function. Meanwhile, the ADC values of parotid and submandibular glands both showed a significant increase, which is in accordance with previous studies (101314). A possible explanation for the ADC trend is that acinar cells of the parotid glands decreased in number as a result of radiation damage (18), and comparable changes have been observed in submandibular tissue (1920). A lower cell density will induce an augmented water diffusivity and consequently an increase in ADC. However, Zhang et al. (15) used relatively low b values (10, 50, 100, 150 s/mm2) and found that the ADC values decreased linearly with impaired salivary gland function after RT. This discrepancy may be attributable to the b values chosen. Lower b values reflect the perfusion and saliva flow in the salivary glands, whereas higher b values approximate true diffusion (11). Decreased blood and saliva flow due to radiation injury may lead to the decrease in ADC obtained from relatively low b values.
The ability of IMRT to avoid unnecessary irradiation of salivary gland tissue, thereby preserving gland function and reducing xerostomia, has been abundantly demonstrated. In a matched case-control study, Jabbari et al. (21) found that xerostomia improved over time after IMRT, but not after conventional RT, and that the potential benefits gained from IMRT were not apparent until 6 months or more after therapy. Lin et al. (22) reported that both xerostomia and quality of life improved significantly during the first year after IMRT. In the present study, xerostomia changed from Grade 2 to Grade 1 in 56.5% patients 1 year after IMRT, suggesting an improvement of dry mouth and recovery of salivary gland function. At the same time, a significant decrease in ADC was found in parotid glands but not in submandibular glands, which were not spared during IMRT. The drop in pADC is thought to reflect the decreased water diffusivity as a result of an increase in the acinar cell number, which indicates functional recovery of the glands. A rat model study has proven that an initial drop in the number of acinar cells was followed by an increase in the late post-irradiation period (120–240 days) (23). In addition, radiation-induced damage in the lower dose range might be reversible to a certain extent, whereas higher doses generally produce a permanent loss of salivary gland function (24). This may explain why the sADC receiving a higher dose than parotid glands in our patients did not recover over time. In our study, the radiation dose to submandibular glands was higher compared to that of parotid glands, perhaps causing greater damage and delayed healing in submandibular glands. This might be an explanation for the sADC in which no change was found during the follow-up after RT.
Diffusion-weighted imaging combined with gustatory stimulation could acquire more valuable information regarding secretory function. In accordance with previous studies (891012), a significant increase in ADC values of unirradiated parotid glands was found after stimulation, which may be due to the active production of new saliva and consequently an increase of free water in the extracellular space after stimulation. A similar response to gustatory stimulation was found after RT, with a longer pTmax. A possible explanation could be that the cells' capacity to produce saliva after IMRT persisted, but radiation damage could lead to acinar cell loss and hampered water secretion, resulting in a lower rate to the peak state.
An exponential relationship between function loss and mean dose has been clearly demonstrated for parotid glands, suggesting that it is essential to respect a certain mean dose threshold to preserve gland function (25). Different thresholds of radiation dose, ranging from 20 Gy to 40 Gy, have been shown in previous studies (252627). In this study, we did not intend to calculate the threshold for salivary glands because all patients underwent IMRT and received a relatively narrow dose range. However, we found that an improvement in xerostomia was observed in patients receiving a mean parotid dose of 38.84 Gy, significantly lower than that in patients without a recovery, and the parotid dose was confirmed as a significant predictor in multivariate analysis, indicating that high-dose radiation exposure to the parotid glands could aggravate the severity of xerostomia. In addition, we found that patients with late Grade 1 xerostomia had a shorter pTmax than those with Grade 2 at 1 week post-RT. This is thought to reflect less damage and more residual function in parotid glands due to lower mean dose in patients with Grade 1 xerostomia. Another difference between the two groups was that the sADC at 1 week post-RT was higher in patients with Grade 1 xerostomia than those with Grade 2, possibly corresponding to the presence of fibrosis and necrosis at different extents and individual response to irradiation, for the submandibular glands in the two groups both received a high dose irradiation in which there was no significant difference between the two groups. In addition, the relationship among ADC values, salivary function, and radiation dose might be applicable within a certain range. Furthermore, the submandibular glands are different from parotid glands in histologic composition, and they contribute about 70% of all saliva in the resting state. Wada et al. (28) reported that submandibular gland hypofunction had the strongest influence on the clinical severity of xerostomia. Therefore, the feasibility of sparing of submandibular glands with IMRT should also be evaluated.
In our opinion, the most interesting findings of the present study were the significant correlations between early ADC-related parameters and late xerostomia grades after IMRT. ADC is expected to vary according to the microstructures or pathophysiologic states of the tissues, even at very early stage of disease (29). It has been used for prediction of treatment response in head and neck cancers (30). Our results indicated that DWI could allow assessment of radiation-induced changes within the salivary glands predicting for late effects.
This study had some limitations. First, this is the first study using DWI to evaluate salivary gland function in both acute and late radiation-induced xerostomia so there is no guideline for interpreting the results. Moreover, the sample size was relatively small, and the range of radiation dose to salivary glands was narrow. A correlation between the dose and changes in ADC of salivary glands could not be made. Future investigations with a larger patient group are required. Second, because of the considerable scan duration of the DWI sequence, we evaluated only stimulated parotid glands that contribute 60–70% of all saliva under stimulated conditions. However, DWI without stimulation may be used as well to evaluate the radiation-induced changes of submandibular glands by comparing ADC values before and after treatment. In addition, the follow-up period was not long enough because it was reported that salivary gland function could still recover up to 5 years after RT (31). Further, chronic autoimmune sialadenitis that is asymptomatic and normal on imaging studies in its early stages should be considered in the follow-up because limited functional reserve can result in a different response. Finally, it was suggested that late radiation damage after partial irradiation may be region-dependent in salivary glands (32). Therefore, it would be interesting to evaluate whether DWI might be used to assess regional radiation effect inside the glands.
In conclusion, our results demonstrated the post-RT changes in ADC of salivary glands and significant correlations between early ADC-related parameters and late xerostomia grades, suggesting DW-MRI with gustatory stimulation could noninvasively evaluate the functional changes of salivary glands following RT, and it seems a useful tool for early prediction of the severity of radiation-induced xerostomia.
References
1. Huguenin PU, Taussky D, Moe K, Meister A, Baumert B, Lütolf UM, et al. Quality of life in patients cured from a carcinoma of the head and neck by radiotherapy: the importance of the target volume. Int J Radiat Oncol Biol Phys. 1999; 45:47–52. PMID: 10477005.

2. Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol. 2001; 61:271–274. PMID: 11730996.

3. Saarilahti K, Kouri M, Collan J, Kangasmäki A, Atula T, Joensuu H, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol. 2006; 78:270–275. PMID: 16564589.

4. Münter MW, Hoffner S, Hof H, Herfarth KK, Haberkorn U, Rudat V, et al. Changes in salivary gland function after radiotherapy of head and neck tumors measured by quantitative pertechnetate scintigraphy: comparison of intensity-modulated radiotherapy and conventional radiation therapy with and without amifostine. Int J Radiat Oncol Biol Phys. 2007; 67:651–659. PMID: 17175118.

5. Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009; 74:1–8. PMID: 19111400.

6. Eisbruch A, Rhodus N, Rosenthal D, Murphy B, Rasch C, Sonis S, et al. How should we measure and report radiotherapy-induced xerostomia? Semin Radiat Oncol. 2003; 13:226–234. PMID: 12903012.

7. Valdés Olmos RA, Keus RB, Takes RP, van Tinteren H, Baris G, Hilgers FJ, et al. Scintigraphic assessment of salivary function and excretion response in radiation-induced injury of the major salivary glands. Cancer. 1994; 73:2886–2893. PMID: 8199984.

8. Habermann CR, Gossrau P, Kooijman H, Graessner J, Cramer MC, Kaul MG, et al. Monitoring of gustatory stimulation of salivary glands by diffusion-weighted MR imaging: comparison of 1.5T and 3T. AJNR Am J Neuroradiol. 2007; 28:1547–1551. PMID: 17846209.

9. Ries T, Arndt C, Regier M, Graessner J, Cramer MC, Reitmeier F, et al. Value of apparent diffusion coefficient calculation before and after gustatory stimulation in the diagnosis of acute or chronic parotitis. Eur Radiol. 2008; 18:2251–2257. PMID: 18458907.

10. Kato H, Kanematsu M, Toida M, Kawaguchi T, Shibata T, Kajita K, et al. Salivary gland function evaluated by diffusion-weighted MR imaging with gustatory stimulation: preliminary results. J Magn Reson Imaging. 2011; 34:904–909. PMID: 21837780.

11. Thoeny HC, De Keyzer F, Boesch C, Hermans R. Diffusion-weighted imaging of the parotid gland: influence of the choice of b-values on the apparent diffusion coefficient value. J Magn Reson Imaging. 2004; 20:786–790. PMID: 15503336.
12. Loimu V, Seppälä T, Kapanen M, Tuomikoski L, Nurmi H, Mäkitie A, et al. Diffusion-weighted magnetic resonance imaging for evaluation of salivary gland function in head and neck cancer patients treated with intensity-modulated radiotherapy. Radiother Oncol. 2017; 122:178–184. PMID: 27475276.

13. Dirix P, De Keyzer F, Vandecaveye V, Stroobants S, Hermans R, Nuyts S. Diffusion-weighted magnetic resonance imaging to evaluate major salivary gland function before and after radiotherapy. Int J Radiat Oncol Biol Phys. 2008; 71:1365–1371. PMID: 18355977.

14. Marzi S, Forina C, Marucci L, Giovinazzo G, Giordano C, Piludu F, et al. Early radiation-induced changes evaluated by intravoxel incoherent motion in the major salivary glands. J Magn Reson Imaging. 2015; 41:974–982. PMID: 24700435.

15. Zhang L, Murata Y, Ishida R, Ohashi I, Yoshimura R, Shibuya H. Functional evaluation with intravoxel incoherent motion echo-planar MRI in irradiated salivary glands: a correlative study with salivary gland scintigraphy. J Magn Reson Imaging. 2001; 14:223–229. PMID: 11536398.

16. Zhang Y, Ou D, Gu Y, He X, Peng W, Mao J, et al. Diffusion-weighted MR imaging of salivary glands with gustatory stimulation: comparison before and after radiotherapy. Acta Radiol. 2013; 54:928–933. PMID: 23821773.

17. Franzén L, Funegård U, Ericson T, Henriksson R. Parotid gland function during and following radiotherapy of malignancies in the head and neck. A consecutive study of salivary flow and patient discomfort. Eur J Cancer. 1992; 28:457–462. PMID: 1591063.
18. Stephens LC, Schultheiss TE, Price RE, Ang KK, Peters LJ. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer. 1991; 67:1539–1543. PMID: 2001542.

19. Zeilstra LJ, Vissink A, Konings AW, Coppes RP. Radiation induced cell loss in rat submandibular gland and its relation to gland function. Int J Radiat Biol. 2000; 76:419–429. PMID: 10757322.
20. Teshima K, Murakami R, Yoshida R, Nakayama H, Hiraki A, Hirai T, et al. Histopathological changes in parotid and submandibular glands of patients treated with preoperative chemoradiation therapy for oral cancer. J Radiat Res. 2012; 53:492–496. PMID: 22485019.

21. Jabbari S, Kim HM, Feng M, Lin A, Tsien C, Elshaikh M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: initial report. Int J Radiat Oncol Biol Phys. 2005; 63:725–731. PMID: 16199308.

22. Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003; 57:61–70. PMID: 12909216.

23. Coppes RP, Zeilstra LJ, Kampinga HH, Konings AW. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br J Cancer. 2001; 85:1055–1063. PMID: 11592779.

24. Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999; 45:577–587. PMID: 10524409.

25. Roesink JM, Schipper M, Busschers W, Raaijmakers CP, Terhaard CH. A comparison of mean parotid gland dose with measures of parotid gland function after radiotherapy for head-and-neck cancer: implications for future trials. Int J Radiat Oncol Biol Phys. 2005; 63:1006–1009. PMID: 15964708.

26. Chambers MS, Garden AS, Rosenthal D, Ahamad A, Schwartz DL, Blanco AI, et al. Intensity-modulated radiotherapy: is xerostomia still prevalent? Curr Oncol Rep. 2005; 7:131–136. PMID: 15717947.

27. Grégoire V, De Neve W, Eisbruch A, Lee N, Van den Weyngaert D, Van Gestel D. Intensity-modulated radiation therapy for head and neck carcinoma. Oncologist. 2007; 12:555–564. PMID: 17522243.

28. Wada A, Uchida N, Yokokawa M, Yoshizako T, Kitagaki H. Radiation-induced xerostomia: objective evaluation of salivary gland injury using MR sialography. AJNR Am J Neuroradiol. 2009; 30:53–58. PMID: 18842755.

29. Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001; 220:621–630. PMID: 11526259.

30. King AD, Chow KK, Yu KH, Mo FK, Yeung DK, Yuan J, et al. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology. 2013; 266:531–538. PMID: 23151830.

31. Braam PM, Roesink JM, Moerland MA, Raaijmakers CP, Schipper M, Terhaard CH. Long-term parotid gland function after radiotherapy. Int J Radiat Oncol Biol Phys. 2005; 62:659–664. PMID: 15936542.

32. Konings AW, Cotteleer F, Faber H, van Luijk P, Meertens H, Coppes RP. Volume effects and region-dependent radiosensitivity of the parotid gland. Int J Radiat Oncol Biol Phys. 2005; 62:1090–1095. PMID: 15990013.

Fig. 1
55-year-old male patient with nasopharyngeal carcinoma before RT.
Axial T2-weighted images and corresponding diffusion-weighted images of parotid (A, B) and submandibular glands (C, D) were obtained. Regions of interest were manually drawn in reference to T2-weighted images, including as much of gland parenchyma as possible. RT = radiotherapy
Fig. 2
Box plots show different ADC levels of salivary glands in all patients before and after RT.
Mean and median are given by dotted and solid lines, respectively (dotted lines, means; solid lines, medians; boundaries of boxes, 25%/75% quintiles; error bars, 10%/90% quintiles; data points, outliers). Graph shows that ADC values of salivary glands increase after RT (p < 0.001). Compared with 1 week post-RT, pADC and pADCmax both decreased at 1 year post-RT (p < 0.001), but sADC shows no significant change (p = 0.581). ADC = apparent diffusion coefficient, pADC = ADC of parotid glands at rest, pADCmax = maximum ADC of parotid glands, sADC = ADC of submandibular glands at rest
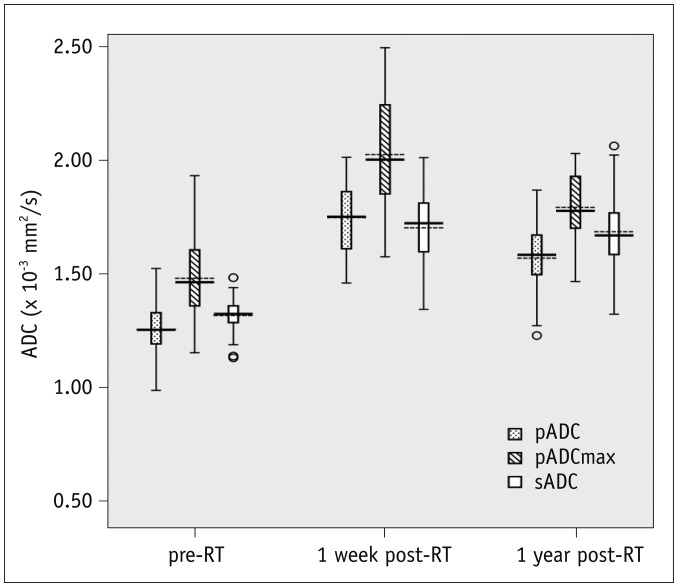
Fig. 3
Bar charts show mean values and 95% of confidence intervals of pTmax after stimulation in patients with different grades of late xerostomia.
Only at 1 week post-RT, two groups show significant differences (p = 0.019). pTmax = Time to peak ADC of parotid glands
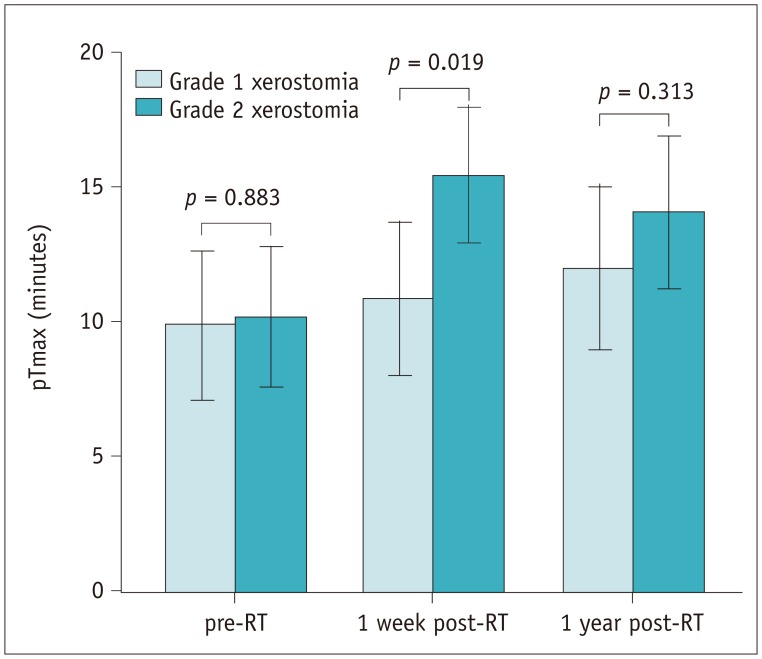
Fig. 4
Box plots show different ADC levels of salivary glands at 1 week post-RT in patients with different grades of late xerostomia.
Mean and median are given by dotted and solid lines, respectively (dotted lines, means; solid lines, medians; boundaries of boxes, 25%/75% quintiles; error bars, 10%/90% quintiles; data points, outliers). Graph shows that sADC is higher in patients with Grade 1 xerostomia than those with Grade 2 (p = 0.013).
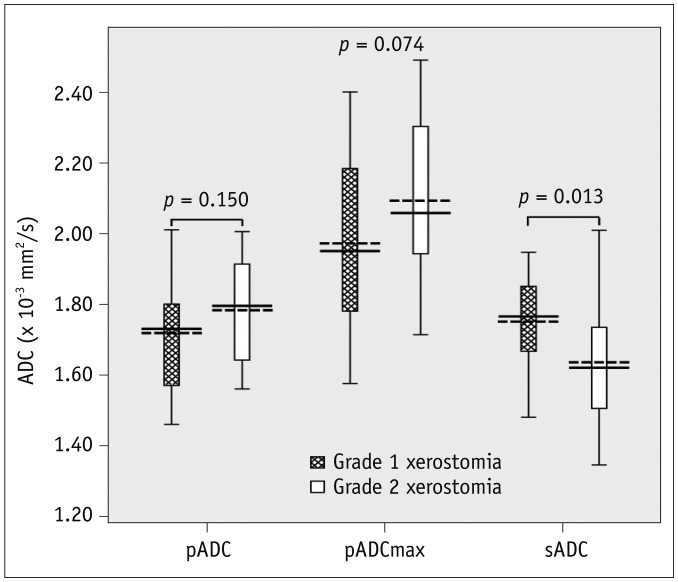
Fig. 5
ADC changes of parotid glands.
Line graphs indicate ADC changes of parotid glands after stimulation at 1 week post-RT in 55-year-old male patient (patient 1) who experienced Grade 2 xerostomia both at 1 week and 1 year post-RT and 56-year-old female patient (patient 2) who had improvement in xerostomia late after RT. pADCmax were lower and pTmax were shorter in patient 2 than in patient 1. sADC were higher in patient 2 (R = 1.81 × 10−3 mm2/s, L = 1.74 × 10−3 mm2/s, not shown) than in patient 1 at 1 week post-RT (R = 1.58 × 10−3 mm2/s, L = 1.60 × 10−3 mm2/s). L = left, R = right
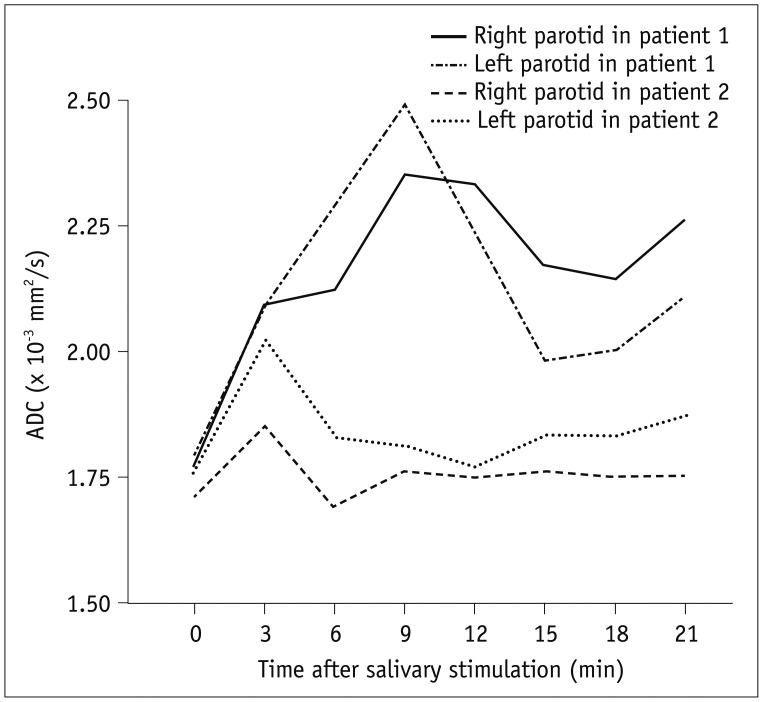
Table 1
Doses of Salivary Glands for All Patients and Subgroups with Different Grades of Late Xerostomia
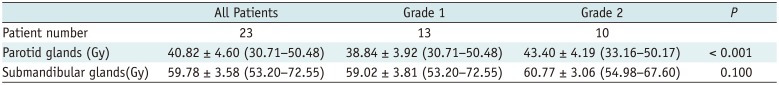
Table 2
Diffusion-Weighted Magnetic Resonance Imaging Findings of Salivary Glands at Different Times before and after RT for Patients Grouped by Clinical Xerostomia at One Year Post-RT (× 10−3 mm2/s)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download