Abstract
Objective
This study's purposes were to determine the yield of repeat direct in-bore magnetic resonance-guided prostate biopsy (MRGB) (MRGB-2) after the first one was found to be negative (MRGB-1), to correlate with clinical parameters, and to present the subgroup analyses of patients with positive repeat biopsies, despite having a negative initial biopsies.
Materials and Methods
We retrospectively included patients with MRGB-2 after a negative MRGB-1 both between January 2006 and August 2016. This study included 62 patients (median age, 63 years; interquartile range [IQR], 58–66 years) with 75 sampled lesions during MRGB-2 left for analysis, and 63 lesions were resampled and 12 new lesions were sampled. Included patients had a prostate specific antigen (PSA) at MRGB-1 of 13 ng/mL (IQR, 5.8–20.0) and a PSA at MRGB-2 of 15 ng/mL (IQR, 9.0–22.5). All anonymized magnetic resonance imaging (MRI) data were retrospectively reassessed according to Prostate Imaging-Reporting and Data System version 2 by two radiologists. Images of MRGB were compared to determine whether the same prostate lesion was biopsied during MRGB-1 and MRGB-2. Descriptive statistics were utilized to determine the yield of clinically significant prostate cancer (csPCa) at MRGB-2. Gleason score of ≥ 3 + 4 was considered csPCa.
Since its introduction, multiparametric magnetic resonance imaging (mpMRI) of the prostate and subsequent targeted biopsy improved the detection of clinically significant prostate cancer (csPCa), without increasing the detection of insignificant PCa when compared to systematic transrectal ultrasound (TRUS)-guided biopsy (123). Reported csPCa detection rates of targeted biopsy (e.g., fusion-guided or direct in-bore magnetic resonance [MR] targeted) in men with a suspicious lesion ranged from 17–52%, with several reasons for this serious range. Some of these reasons are the diverse definitions for csPCa, the various thresholds from where a lesion is biopsied, the ranging MRI protocols and the differences in study protocols being either prospective or retrospective (1456789). Detection rates for any PCa even ranges from 22–79% in men with lesions detected on prebiopsy mpMRI (461011). Contrariwise, biopsy did not reveal any PCa in 21–78% of those men, although they had a suspicious lesion seen on mpMRI. In our institution, such patients, in whom direct in-bore MR-guided biopsy (MRGB) was negative despite a suspicious lesion, are frequently followed by measuring the serum prostate specific antigen (PSA) and if required, obtaining another mpMRI or even repeating the MRGB. Consequently, additional costs are being made and patients are subjected to risks and the inconvenience associated with it.
Previously, Chelluri et al. (12) described their findings in 90 men having a second MRI-TRUS fusion guided biopsy after the first one was negative, yielding 6.0% Gleason score (GS) ≥ 3 + 4 lesions. In addition, recently Costa et al. (13) presented their results yielding 40% of intermediate and high risk cancers with repeat targeted biopsies or surgeries in 38 highly suspicious lesions with an initial negative MRI-TRUS fusion targeted prostate biopsy.
To our knowledge, the yield of repeated MRGB in this particular clinical scenario is not well established. This is especially interesting as direct in-bore MR targeted biopsy is still the most accurate prostate biopsy technique (71415). Therefore, the aim of our paper was to determine the yield of repeat MRGB (MRGB-2) in patients having a negative first one (MRGB-1) and to correlate with clinical parameters and to present subgroup analyses of patients with positive repeat biopsies despite having negative initial biopsies.
This retrospective study was approved by our Institutional Review Board with a waiver of informed consent (2016-2767).
The Picture Archiving and Communication System (PACS) data was searched for patients who had at least two consecutive MRGBs in our institution between January 2006 and August 2016. Patients were excluded if any PCa was detected before MRGB-1 or between MRGB-1 and MRGB-2. Thus, included patients did not have a biopsy proven PCa before MRGB-1, had a negative MRGB-1, had a lesion which was rebiopsied during MRGB-2 and had an mpMRI before MRGB-1 (Fig. 1). As a consequence of these criteria, we could not provide the results of the overall detection rates during MRGB-1 or MRGB-2.
In 81 patients, an MRGB was performed twice in the same patient between January 2006 and August 2016. Of those patients, 19 were excluded because they had a GS 3 + 3 after MRGB-1.
After exclusion, 62 patients were included with 98 lesions biopsied during MRGB-1. Seven patients were biopsy naïve prior to MRGB-1. During MRGB-2, 63 of those lesions were rebiopsied and 12 new lesions were biopsied. In 3 out of these 12 new biopsied lesions, csPCa was detected. Overall, 35 lesions biopsied during MRGB-1 were not rebiopsied during MRGB-2 (Fig. 1), because they were not visible anymore or not suspicious for csPCa at mpMRI-2 (i.e., Prostate Imaging-Reporting and Data System [PI-RADS] classification 1 or 2). However, because of a lack of pathologic confirmation in those lesions, we did not evaluate this any further. Patient characteristics can be found in Table 1.
MRI was performed on a 3T MR-scanner (Skyra, Siemens, Erlangen, Germany), with a pelvic phased-array coil. In four patients an endorectal coil was utilized during first mpMRI instead of a pelvic phased-array coil. In all patients, tri-planar anatomical T2-weighted images (T2WI), axial dynamic contrast-enhanced images (DCE) and axial diffusion-weighted images (DWI) were usually obtained. However, because of the large time span in which patients were included, there is a slight variation in mpMRI technical specifications. A typical T2WI sequence had a repetition time (TR) of 3540 ms (range, 3000–7170), an echo time (TE) of 104 ms (range, 99–121), a flip angle of 120 degrees (range, 120–170), a turbo factor of 15 (range, 15–25), a matrix of 320 × 320 pixels (range, 224 × 448–384 × 384), and a slice thickness of 3 mm (range, 3–4), with 15–27 slices needed to cover the entire prostate. DWI typically consisted of a calculated apparent diffusion coefficient map and multiple b-values (0, 50, 500, 800, 1400), a matrix of 128 × 108 pixels (range, 100 × 100–144 × 192) with a slice thickness of 4 mm (range, 3–5) with 20 (range, 15–23) slices to cover the prostate. DCE was obtained using 15 mL gadoterate meglumine (Dotarem; Guerbet LCC, Bloomington, IN, USA) with an injection rate of 2.5 mL/s followed by a 20 mL NaCl flush, an acquisition time of 2.5 minutes (range, 2–6) and a TR of 32 ms (range, 32–44). Patient characteristics are listed in Table 1, and follow up after MRGB-2 until September 15, 2017 were acquired from hospital records by one observer.
During MRGB, patients were placed in the prone position with an MR-compatible needle guide rectally inserted. The needle guide was attached to a biopsy device DynaTRIM (Invivo corp., Gainesville, FL, USA). During the MRGB session, axial T2WI and axial DWI were obtained to reproduce a lesion's location. The needle guide was manually positioned using true fast imaging with steady-state free precession (TRUFI; Skyra, Siemens) images. An MR-compatible 18-gauge biopsy gun was used to obtain biopsy cores. The lengths of the obtained cores were 17 mm. Usually, two cores per lesion are obtained. Immediately after the biopsy, with the biopsy needle still inserted, TRUFI images in two directions were obtained to confirm biopsy position. The position of the needle was assessed by one of the radiologists experienced in prostate MR readings. In case there is uncertainty about the accuracy of a needle, a third biopsy core was obtained. The ideal location of the needle is when the tip is piercing through the lesion as the tip of the needle does not obtain tissue. During MRGB, no anesthetics were utilized. Obtained pathological tissue was interpreted by dedicated pathologists with 20–30 years of experience in prostate specimen analysis. Procedure time for MRGB was typically 45–60 minutes.
Nowadays, in our institution, the threshold for biopsying a lesion is PI-RADS ≥ 3. However, as patients had mpMRI and MRGB before 2012, not every lesion included was scored according to PI-RADS. Therefore, for this study, mpMRI images were anonymized and they were reassessed according to the PI-RADS version 2 by two radiologists with 5 and 17 years of experience in prostate MR readings (respectively) (16). Disagreements were resolved by consulting a third reader with 12 years of experience in prostate MR readings. MRGB images were used to assess whether the same lesion was rebiopsied during MRGB-2. Only the lesions which were biopsied twice were reassessed. In case other lesions were detected, we did not evaluate them in this study. Radiologists were blinded for pathological outcomes of MRGB-2 but were aware of the negative outcomes of MRGB-1. Lesion volume was calculated by the ellipsoid formula ([left-right × anterior-posterior × cranial-caudal diameter] × π/6). The averages of the calculated volumes were used in the analyses.
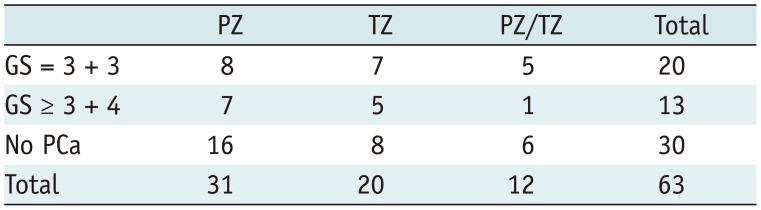
During MRGB-2, 16 of 75 (21%) biopsied lesions resulted in csPCa. Of 63 rebiopsied lesions, 33 (52%) showed any PCa and 13 (21%) csPCa. Six lesions harbored GS 3 + 4, two GS 4 + 3, two GS 3 + 5, one GS 5 + 3 and two GS 4 + 5. The median volume of lesions with detected csPCa at repeat biopsy was 0.67 mL (0.39–2.10) and the median increase in volume of these lesions was 0.43 mL (interquartile range, −0.08–0.70). Lesion location was represented in Table 2.
In two patients, csPCa was detected (GS 4 + 5 and 3 + 5) at repeat biopsy, while the volume of the lesion decreased between MRGB-1 and MRGB-2. In both patients, two radiologists agreed on the accuracy of MRGB-1 hitting the lesion properly. Figures 2,3,4,5 are examples of patients with csPCa findings at repeat biopsy.
At initial mpMRI, 32 lesions scored PI-RADS 2 after consulting the third reader. Five lesions scored PI-RADS 3. PI-RADS 4 was scored in 19 patients. The remaining 7 patients scored PI-RADS 5 at initial mpMRI. Detection rates for csPCa was 19% (6/32) in PI-RADS 2, 20% (1/5) in PI-RADS 3, 11% (2/19) in PI-RADS 4 and 57% (4/7) in PI-RADS 5, respectively. Any PCa was detected in 41% (13/32), 80% (4/5), 53% (10/19), and 86% (6/7) in PI-RADS 2, 3, 4, and 5, respectively
A moderate interobserver agreement between both radiologists for the PI-RADS classification for lesions at mpMRI was reached with a weighted kappa value of 0.57 (95% confidence interval, 0.36–0.78). In none of the patients with a decreasing PSA between MRGB-1 and MRGB-2, csPCa was detected (Fig. 6). In patients with repeat biopsies, MRGB-1 was mostly performed in years 2010 and 2011 (n = 34) with 7 lesions resulting in csPCa at MRGB-2. MRGB-1 was never performed in year 2016 (Fig. 7).
Three patients with negative MRGB-2 had another MRGB (hereafter, MRGB-3). In none of them PCa was detected. Their reassessed PI-RADS scores were PI-RADS 2, 4, and 5. In nine patients an mpMRI after MRGB-2 was obtained, those were assessed as being PI-RADS 2, no MRGB-3 was performed in those patients. In six patients no additional mpMRI was performed, because the PSA decreased after MRGB-2. In the remaining 45 patients, follow up data was not available.
To our knowledge, this study displayed the results of the first cohort of patients having repeat MRGB with negative first biopsies collected in more than ten years. We demonstrated that it might be beneficial to repeat the MRGB in case there is a continuing suspicion for having csPCa, despite a negative first MRGB, as csPCa is detected in 21% of 75 lesions biopsied at MRGB-2. With a csPCa detection rate of again 21% (n = 13/63) for resampling the exact same lesion, we also demonstrated that it might be beneficial to target a lesion which was histologically proven to be negative on prior MRGB.
With increasingly performed targeted biopsies, urologists are more often faced with patients with a suspicious lesion detected on mpMRI and a negative pathology outcome for that suspicious lesion. Yet, there is no guideline for follow up in such patients. Our study demonstrated that it might be beneficial for some patients to undergo rebiopsy such a lesion. This underlines the importance of follow up for some patients.
Studies about a repeat biopsy or surgery after MRI-TRUS fusion targeted biopsy showed similar results. However, a head-to-head comparison is quite difficult due to the low number of patients in those and in our study (1213). The lengthy time range of our study allows us to raise the question whether our findings still apply in today's practice. Therefore we reevaluated all biopsied lesions and we demonstrated that csPCa detection did not evidently change the course of the years in patients with repeat biopsy.
In retrospect, almost half of our cohort scored PI-RADS 2. This might be caused by an increase in experience we have in evaluating prostate MRI over the years. However, even within the cohort of patients with a reassessed PI-RADS 2, almost 19% (n = 6/32) harbored csPCa at repeat biopsy. This is remarkable, as a comparable csPCa detection rate was seen in this study in patients with PI-RADS ≥ 3 (23%, 7/31). Moreover, detection rates for PI-RADS 4 and 5 described in the literature are quite higher (17181920). This can have several causes, for example, the relatively small sample size of this study making a comparison of detection rates with those of other studies harder. Also, the lesions are already presampled making the yield of the rebiopsy poorer. Furthermore, involved radiologists may be biased by the knowledge that some of the biopsy results after MRGB-2 were positive for csPCa and that a first biopsy in the same lesion was negative. However, they were not aware of which and how many lesions were positive for csPCa. Also, the range of used mpMRI sequences might cause difficulties in the reassessment of the obtained images.
Intriguing are the two lesions with a decrease in volume and yet csPCa at repeat biopsy, as these findings are contrary to one's expectation. In both lesions, the radiologists rated the biopsies as accurate samplings of the lesions during MRGB-1. This can occur, for example, in case of surrounding tissue reaction which could be regressive in control scans and thus cause a false decrease in lesion diameter (21). Further, we found that the time between MRGB-1 and MRGB-2 and PSA changes does not seems to influence the biopsy outcome of MRGB-2. Recommendations on timing of follow up in patients with change in PSA are therefore not possible. It appears that a rise in PSA, even a rapid increase in a relatively short period, seems not to be very helpful in selecting patients who will benefit from repeat biopsy. In an important amount of patients in our cohort with a rapid increase in PSA, csPCa was not detected during MRGB-2. On the other hand, none of the patients with a decreasing PSA had csPCa when resampling the same lesion. Also, timing of MRGB-1 does not seem to influence the results. The total number of performed MRGB-1 was highest in years 2010 and 2011, and also MRGB-1 of lesions resulting in csPCa at MRGB-2 was mostly performed within that time period. This might be explained by the fact that in our institution the total number of performed MRGBs was highest in that period.
One explanation for the detection of csPCa during MRGB-2 after a first session was negative may be the limitation of the biopsy technique. Although confirmation scans are made with the biopsy needle left in position, it remains difficult to assess the intralesional needle placement. Second, it may be explained by the biological progression. However, with a median time between MRGB-1 and MRGB-2 of 11 months in patients with positive MRGB-2, it is not very likely.
The most important limitation of our study was the long time range in which we included patients. Unfortunately, it was necessary to include a reasonable amount of patients. In a period of more than ten years, there are only 62 patients who had a repeat MRGB after an initial negative MRGB in our hospital. Unfortunately, doubling our sample size would thus probably have taken another ten years. Apparently, urologists and patients are not inclined to have another MRGB after the first one was negative. This can be well explained by the additional patient burden, the costs and the lower probability to detect a csPCa at repeat biopsy. A limitation of this large time span is the introduction of a learning curve. Nowadays, we have a lot more experience in both mpMRI readings and targeted biopsiescompared to ten years ago. Several studies showed the importance of experience in prostate MR reading and the method of biopsy acquisition (722). To minimize this limitation, we reassessed all lesions according to PI-RADS version 2. Also, we assessed whether the biopsy needle accurately targeted the suspicious lesion. Due to the small sample size, we were not able to provide predictors for a positive repeat MRGB. Further, selection bias will be introduced in this study as we only chose resampled lesions. Included patients were rebiopsied, because there was some reason to believe that patients had csPCa regardless of the negative findings during initial MRGB.
Also, a recurring limitation of all biopsy accuracy studies is the lack of a gold standard, for example, transperineal template prostate mapping or final radical prostatectomy. It would be extremely interesting to know whether a negative biopsy outcome is truly negative. Also, we only performed targeted biopsy without an additional systematic biopsy. As some studies reported csPCa detection rates up to 10% using systematic biopsy, which would be missed in a targeted only approach. Some advocate to perform systematic biopsy in addition to targeted biopsy (12223). Nonetheless, this study was only focusing on the yield of repeat targeted biopsy. It solely provides information on the utility of targeting a lesion again while it was negative for PCa on previous targeted biopsy. We hereby accept the chance that when we missed a lesion during the first biopsy, we might miss it again during repeat biopsy. In our institution, we try to minimize this chance by targeting each suspicious lesion at least twice. This approach, however, is debated as Schimmöller and colleagues demonstrated that there is limited benefit of targeting a lesion twice in the same session (24).
Based on our results, taking the limitations into consideration, we can conclude that an important amount of patients might benefit from repeat biopsy after a negative MRGB. Disagreement between mpMRI lesion characteristics and the pathology should be evaluated carefully. Based on this study we cannot suggest which patients will benefit. Therefore, further study is warranted to establish predictors for a positive repeat targeted biopsy.
References
1. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015; 313:390–397. PMID: 25626035.

2. Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010; 183:520–527. PMID: 20006859.

3. Fütterer JJ. Multiparametric MRI in the detection of clinically significant prostate cancer. Korean J Radiol. 2017; 18:597–606. PMID: 28670154.

4. Meng X, Rosenkrantz AB, Mendhiratta N, Fenstermaker M, Huang R, Wysock JS, et al. Relationship between prebiopsy multiparametric magnetic resonance imaging (MRI), biopsy indication, and MRI-ultrasound fusion-targeted prostate biopsy outcomes. Eur Urol. 2016; 69:512–517. PMID: 26112001.

5. Baco E, Rud E, Eri LM, Moen G, Vlatkovic L, Svindland A, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol. 2016; 69:149–156. PMID: 25862143.

6. Venderink W, van Luijtelaar A, Bomers JG, van der Leest M, Hulsbergen-van de Kaa C, Barentsz JO, et al. Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur Urol. 2017; 2. 28. [Epub]. DOI: 10.1016/j.eururo.2017.02.021.

7. Wegelin O, van Melick HHE, Hooft L, Bosch JLHR, Reitsma HB, Barentsz JO, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. 2017; 71:517–531. PMID: 27568655.

8. Arsov C, Rabenalt R, Blondin D, Quentin M, Hiester A, Godehardt E, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015; 68:713–720. PMID: 26116294.

9. Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017; 389:815–822. PMID: 28110982.

10. Delongchamps NB, Peyromaure M, Schull A, Beuvon F, Bouazza N, Flam T, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013; 189:493–499. PMID: 22982424.

11. Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013; 189:86–91. PMID: 23158413.

12. Chelluri R, Kilchevsky A, George AK, Sidana A, Frye TP, Su D, et al. Prostate cancer diagnosis on repeat magnetic resonance imaging-transrectal ultrasound fusion biopsy of benign lesions: recommendations for repeat sampling. J Urol. 2016; 196:62–67. PMID: 26880408.

13. Costa DN, Kay FU, Pedrosa I, Kolski L, Lotan Y, Roehrborn CG, et al. An initial negative round of targeted biopsies in men with highly suspicious multiparametric magnetic resonance findings does not exclude clinically significant prostate cancer-preliminary experience. Urol Oncol. 2017; 35:149.e15–149.e21.

14. Schimmöller L, Blondin D, Arsov C, Rabenalt R, Albers P, Antoch G, et al. MRI-guided in-bore biopsy: differences between prostate cancer detection and localization in primary and secondary biopsy settings. AJR Am J Roentgenol. 2016; 206:92–99. PMID: 26700339.

15. Penzkofer T, Tuncali K, Fedorov A, Song SE, Tokuda J, Fennessy FM, et al. Transperineal in-bore 3-T MR imaging-guided prostate biopsy: a prospective clinical observational study. Radiology. 2015; 274:170–180. PMID: 25222067.

16. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol. 2016; 69:16–40. PMID: 26427566.

17. Roethke MC, Kuru TH, Schultze S, Tichy D, Kopp-Schneider A, Fenchel M, et al. Evaluation of the ESUR PI-RADS scoring system for multiparametric MRI of the prostate with targeted MR/TRUS fusion-guided biopsy at 3.0 Tesla. Eur Radiol. 2014; 24:344–352. PMID: 24196383.

18. Osses DF, van Asten JJ, Kieft GJ, Tijsterman JD. Prostate cancer detection rates of magnetic resonance imaging-guided prostate biopsy related to Prostate Imaging Reporting and Data System Score. World J Urol. 2017; 35:207–212. PMID: 27287889.

19. Felker ER, Lee-Felker SA, Feller J, Margolis DJ, Lu DS, Princenthal R, et al. In-bore magnetic resonance-guided transrectal biopsy for the detection of clinically significant prostate cancer. Abdom Radiol (NY). 2016; 41:954–962. PMID: 27118268.

20. Pokorny MR, de Rooij M, Duncan E, Schröder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014; 66:22–29. PMID: 24666839.

21. Le Nobin J, Orczyk C, Deng FM, Melamed J, Rusinek H, Taneja SS, et al. Prostate tumour volumes: evaluation of the agreement between magnetic resonance imaging and histology using novel co-registration software. BJU Int. 2014; 114(6b):E105–E112. PMID: 24673731.

22. Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015; 68:1045–1053. PMID: 25656808.

23. Abd-Alazeez M, Kirkham A, Ahmed HU, Arya M, Anastasiadis E, Charman SC, et al. Performance of multiparametric MRI in men at risk of prostate cancer before the first biopsy: a paired validating cohort study using template prostate mapping biopsies as the reference standard. Prostate Cancer Prostatic Dis. 2014; 17:40–46. PMID: 24126797.

24. Schimmöller L, Quentin M, Blondin D, Dietzel F, Hiester A, Schleich C, et al. Targeted MRI-guided prostate biopsy: are two biopsy cores per MRI-lesion required? Eur Radiol. 2016; 26:3858–3864. PMID: 26920391.

Fig. 1
Flowchart and in- and exclusion criteria of patients with repeat MRGB in our institution.
cs = clinically significant, GS = Gleason score, MRGB = direct in-bore magnetic resonance imaging-guided biopsy, PCa = prostate cancer
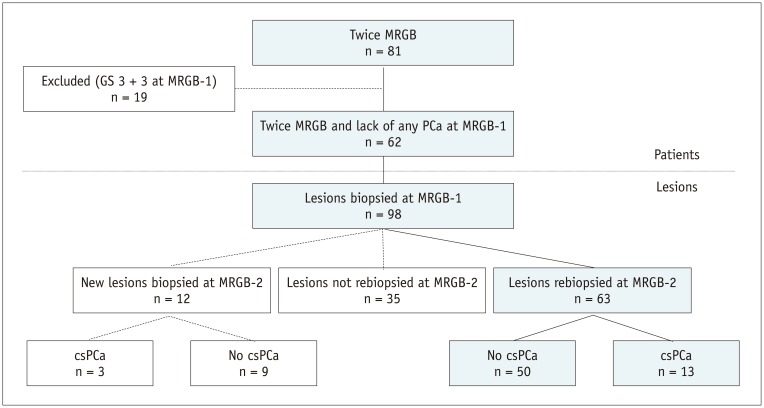
Fig. 2
Example of decreasing lesion volume.
68-year-old patient with PSA of 9.9 ng/mL having mpMRI-1 and subsequent MRGB-1 of lesion which was retrospectively scored PI-RADS 2 (A-D). Maximal lesion diameter was 15 mm. After 11 months, his PSA increased to 12.6 ng/mL, while lesion volume decreased 0.23 mL. Maximum lesion diameter was unchanged. At mpMRI-2, lesion still scored PI-RADS 2 (E-H). During MRGB-2 (Fig. 3) GS 3 + 5 csPCa was detected. (A, E) axial T2WI, (B, F) calculated axial ADC map, (C, G) axial DWI, (D, H) color map representing dynamic contrast enhancement images. ADC = apparent diffusion coefficient, DWI = diffusion-weighted images, mpMRI = multiparametric magnetic resonance imaging, PI-RADS = Prostate Imaging-Reporting and Data System, PSA = prostate specific antigen, T2WI = T2-weighted images
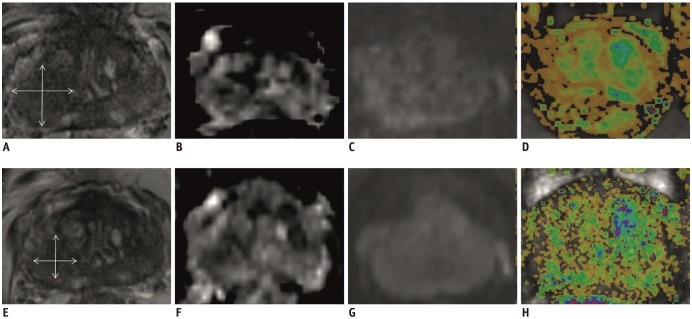
Fig. 3
Confirmation scan of biopsy needle during MRGB of patient represented in Figure 2.
A, B. Respectively axial and sagittal images of MRGB-1. C, D. Respectively axial and sagittal images of MRGB-2. Needle was assumed to properly sample lesion.
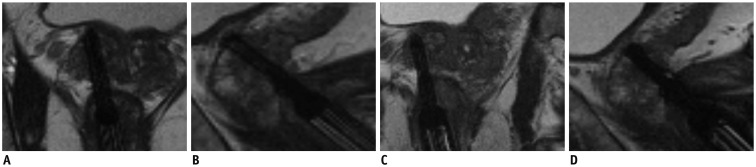
Fig. 4
Example of decreasing lesion volume.
52-year-old patient with PSA of 3.0 ng/mL having mpMRI-1 and subsequent MRGB-1 of lesion which was retrospectively scored PI-RADS 3 (A-D). Maximal lesion diameter was 9 mm. After 17 months, his PSA increased to 8.6 ng/mL and lesion volume increased 0.44 mL. Maximal lesion diameter increased to 13.5 mm. At mpMRI-2, lesion was scored PI-RADS 4 (E-H). During MRGB-2 (Fig. 5) GS 3 + 4 csPCa was detected. (A, E) axial T2WI, (B, F) calculated axial ADC map, (C, G) axial DWI, (D, H) color map representing dynamic contrast enhancement images.
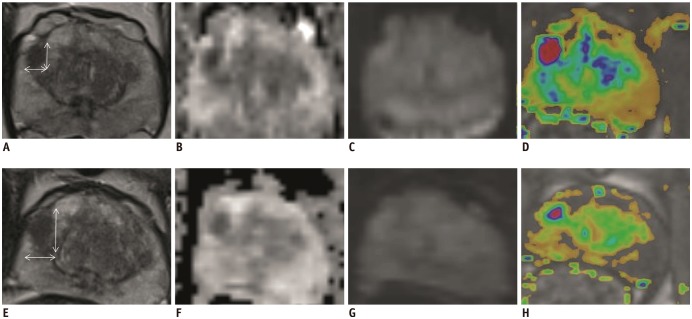
Fig. 5
Confirmation scan of biopsy needle during MRGB of patient represented in Figure 4.
A, B. Respectively axial and sagittal images of MRGB-1. C, D. Respectively axial and sagittal images of MRGB-2. Needle was assumed to properly sample lesion.
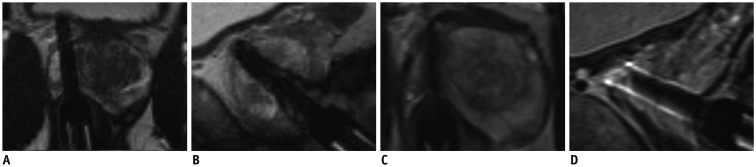
Fig. 6
Detection of csPCa correlated to time between MRGB-1 and MRGB-2 and to change in PSA.
Triangles are representing lesions with csPCa at MRGB-2 and diamonds lesions without csPCa at MRGB-2.
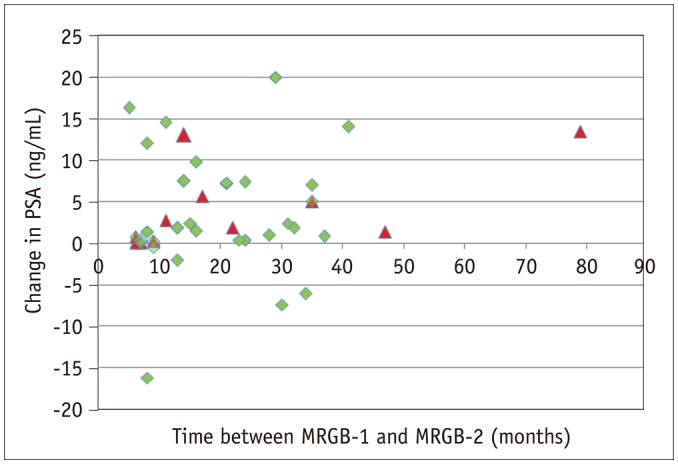
Fig. 7
Timing of MRGB-1 correlated to number of lesions with csPCa detected during MRGB-2.
In patients with repeat biopsy, MRGB-1 was never performed in year 2016.
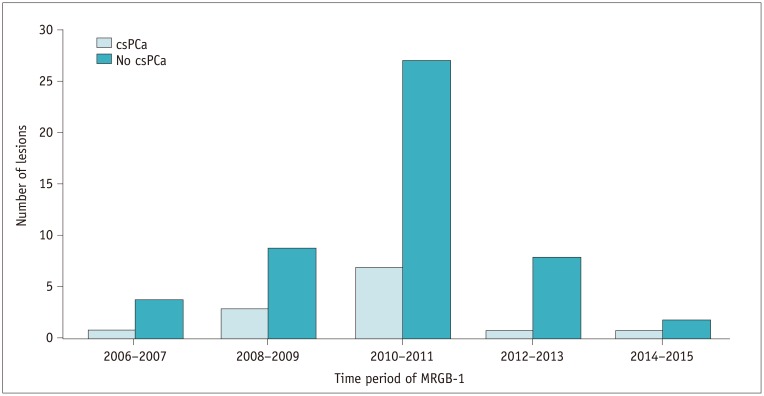
Table 1
Patient and Lesion Characteristics of Included Patients
Analysis was done on per lesion basis. In seven patients two lesions were rebiopsied, so their age, prior No. of TRUS biopsy and PSA is twice included in this analysis. csPCa = clinically significant prostate cancer, IQR = interquartile range, MRGB = direct in-bore magnetic resonance imaging guided biopsy, PI-RADS = Prostate Imaging-Reporting and Data System, PSA = prostate-specific antigen, TRUS = transrectal ultrasound guided biopsy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download