Abstract
Objective
The study aimed to evaluate the contributions of levator ani muscle (LAM) injury, vesical neck movement, urethral length and mobility, and urethral sphincter dysfunction observed on magnetic resonance imaging (MRI) towards stress urinary incontinence (SUI) after vaginal delivery.
Materials and Methods
Fifty primiparous women after 6 months of delivery (15 with SUI and 35 without) and 35 nulliparous as continent controls underwent MRI at rest and Valsalva maneuver. A published levator ani scoring system was used to characterize morphological changes of LAM. The severity of the injury was divided into three categories as none, minor, and major. A series of common parameters including levator plate angle, iliococcygeal angle, and levator hiatus were used to describe the functional conditions of LAM. Urethral mobility was defined based on the rotation of the urethra between Valsalva and rest status. Vesical neck movement was evaluated by its distance to the pubococcygeal line. Urethral sphincter dysfunction was defined as the widening of the proximal urethra and/or funneling at the urethrovesical junction during Valsalva.
Results
Primiparous incontinent (PI) women had additional major levator ani defects (33.3% vs. 17.1%) while less minor defects (0.7% vs. 31.4%) than primiparous continent (PC) women. Vesical neck downward movement in PI women was more obvious than PC women (28.5 mm vs. 24.2 mm, p = 0.006). Urethral mobility was more active in primiparous women than in nulliparous continent controls (57.4 vs. 52.4), whereas no difference was observed on urethral mobility in the primiparous group (p = 0.25). Urethral sphincter dysfunction and funneling were present in 80% of PI women versus 22.9% in PC women (p < 0.001).
Conclusion
The MRI findings revealed that de novo SUI was associated with major LAM injury, vesical neck downward movement as well as urethral sphincter dysfunction. Vesical neck funneling on sagittal images can be treated as a valuable predictor for SUI. The intervention for the PI should focus on the elevation of vesical neck, rehabilitation of LAM as well as recovery of the urethral sphincter muscle.
Stress urinary incontinence (SUI) is defined as an involuntary urine leakage during a sudden increase in intraabdominal pressure such as coughing, sneezing, laughing or exercise. It is the most common pelvic floor dysfunction linked to birth, especially the vaginal delivery (1). In a population-based study of 15307 women, Rortveit et al. (2) found that 14.7% of parous women had symptoms of SUI, while 4.7% of nulliparous women did. Currently, a list of hypothesis for SUI has been put forward, which includes the urethral sphincter deficiency, urethral support failure, and urethral hypermobility (345). Previous studies on SUI after birth focused on vesical neck movement only (67) or combination of urethral function and mobility (8). Functional parameters such as the maximum urethral closure pressure, urethral mobility, and the vesical neck movement were assessed by traditional methods employing urodynamics, Q-tip, and ultrasound (6789). All these procedures provide incomplete information and were dependant on the experience and subjectivity of the examiner.
Magnetic Resonance Imaging (MRI) offers a new approach to assess not only detailed morphological information but also simultaneous functional assessment. The high-resolution static MRI provides detailed information about anatomical changes in the levator ani muscle (LAM) which supports the anterior vaginal wall and urethra. The fast-acquisition dynamic MRI can be used for functional assessment by allowing for the real-time continuous imaging of the vesical neck movement, rotation of urethra, and morphological changes of the urethrovesical junction.
The present study was aimed to determine the LAM injury, vesical neck downward movement, urethral length and mobility, and urethral sphincter dysfunction, which contributes towards de novo SUI in the primiparous women. Furthermore, the comparison between the primiparous continent (PC) women and nulliparous continent women was also done to determine the effect of pregnancy and vaginal birth on SUI.
All procedures were approved by the Institutional Review Board of our hospital. Primiparous women which experienced vaginal delivery 6 months ago in our hospital were enrolled by reviewing their delivery records between June 1, 2014, and January 1, 2017. Subjects were excluded if they experienced multi-times of delivery before or delivered only once but delivered at 35 weeks or less weeks during pregnancy. Telephone calls were made to the eligible subjects about participation. Fifty women (mean age, 27.2 ± 2.5 years; age range, 22–31 years) were enrolled in the study. Fifteen women with self-reported stress incontinence beginning from postpartum and still persistent were classified under the incontinent group whilst the other thirty-five women without symptoms were classified under the continent group. The women included in the incontinent group were required to be continent prior to delivery, experience incontinence symptoms after delivery, and the symptom should be persistent at the time of testing. Also, they were required to demonstrate the finding of stress incontinence during a physical examination. The postpartum evaluation was done 6 months later since the recovery of the uterus, healing of muscles and fascia occurs during this period (8). Thirty-five nulliparous women were recruited through advertisement as controls to determine the changes related to vaginal delivery. Signed informed consent was obtained from each subject prior to the examination.
All subjects were asked to empty their bladder and rectum an hour before the examinations which resulted in the suitable amount of bladder filling at the time of examinations. The static and dynamic MRI was performed using a 3T MR scanner (Magnetom Trio; Siemens Healthcare, Erlangen, Germany). Subjects were examined in the supine position with a 32-channel body matrix array coil. A wedge pad was placed under the knee for assisting the Valsalva maneuver. The static MRI included three consecutive T2-weighted, and turbo spin-echo sequences (repetition time [TR], 4110 ms; echo time [TE], 102 ms; matrix, 320 × 256; field of view [FOV], 260 mm; section thickness, 3 mm; voxel, 0.6 × 0.5 × 3.0 mm; averages 2 times) and datasets were acquired on sagittal, coronal, and transverse planes at rest.
The dynamic MRI consisted of a T2-weighted, single-slice True Fast Imaging with Steady-state Procession (True-FISP) sequence (TR, 4.17 ms; TE, 2.09 ms; flip angle, 70 degree; matrix, 192 × 192; FOV, 280 mm; voxel, 1.6 × 1.6 × 5.0 mm; a total of 70 measurements every 37 seconds) on sagittal, coronal, and transverse planes. The subjects were asked to relax the pelvic floor muscle, contract them slowly to the maximum, and then relax and gradually increase the intraabdominal pressure by straining to do the Valsalva maneuver during the examinations (Supplementary Movie 1 in the online-only Data Supplement). The procedure was repeated two to three times till the satisfactory images were acquired. In True-FISP sequence, the magnetic susceptibility artifact is likely to occur at the interface when the rectum is filled with gas, thus additional Half-Fourier-acquisition-single-shot (HASTE) sequence (TR, 2000 ms; TE, 91 ms; matrix, 192 × 192; FOV, 280 mm; voxel, 1.2 × 0.9 × 4.0 mm) which allows for higher soft-tissue contrast and longer acquisition time but less magnetic susceptibility artifact was used in these cases. Since we had prior information about the pelvic floor from previous True-FISP sequence images, only 6–8 slices next to the ideal measured slice were scanned using HASTE sequence. The subjects were asked to strain to the maximum and then hold during the HASTE scans (about 12–16 secodns). The time of total pelvic floor examination varied from 15 minutes to 20 minutes.
The acquired images were evaluated on AW 4.2 Workstation (GE Healthcare, Fairfield, CT, USA). Two radiologists (with more than 5 years of experiences on abdominal imaging) reviewed the images in consensus.
Levator ani muscle injury could be assessed quantitatively by assessing either morphological or functional changes. The morphological changes could be assessed by LAM scoring system proposed by DeLancey et al. (10) for birth-related injury. The bilateral muscles were scored separately. Score as “0” indicated invisible damage; “1” if less than half of muscle was lost, “2” if more than half of muscle was lost; and “3” if the origin of insertion part was disrupted. The bilateral muscles were categorized from 0 to 6: score as 0 indicated no defect, score as 1–3 indicated a minor defect, and score as 4–6 indicated a major defect. A unilateral score of 3, as well as a bilateral score of 4–6, indicated major defect (Fig. 1).
Since LAM scoring system focused on the assessment of pubovisceral muscle, a series of parameters such as levator plate angle (LPA), iliococcygeal angle, and levator hiatus were chosen to comprehensively describe the functional conditions of the LAM injury. All the parameters were acquired during Valsalva maneuver. The iliococcygeal angle was measured as the angle between the iliococcygeal muscle and the horizontal plane of the pelvis (the line joining the bilateral bony markers such as the ischial tuberosity or femoral head) on coronal planes. The LPA was measured as the angle between the levator plate and the horizontal line. The levator hiatus was defined as the area with anteriorly the inferior of the pubis, posteriorly the inner aspect of puborectal muscle at the anorectal angle, and bilaterally the pubovisceral muscles (Fig. 2).
The normal position of the urethra was shown as a ventral concavity because it curved behind the pubis. The length of the urethra was measured on the sagittal plane as the distance from the internal urinary meatus to the distal part of the perineal membrane where the urethra inserts (Fig. 3A). Standard assessment of urethral angle was quantified by measuring the angle from the vertical axis during rest position on the sagittal plane. For measuring the urethral angle during Valsalva, the tangent line was used when the urethra manifested as slightly rotation around the pubic symphysis and the connection from the internal to external orifice was used when the rotation of the urethra was obviously revealed. The urethral mobility was defined as the difference between the rest and Valsalva status (Fig. 3B, C). The function of urethral sphincter muscle was evaluated by the shape of the urethrovesical junction during straining. The widening of the proximal urethra and funneling at the vesical neck was defined as the implication of urethral sphincter dysfunction (Fig. 4).
The locations of the vesical neck at rest and Valsalva were measured as the vertical distance to the pubococcygeal line (PCL), and the movement was defined as the difference between the rest and Valsalva status (Fig. 3D, E).
All data were analyzed using SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL, USA). Continuous outcomes with normal distribution were given as mean ± standard deviation. For continuous variables related to delivery or incontinence, independent-sample t tests were used to compare the PC group with control groups, and both the primiparous groups. Chi-square statistics from two-way contingency tables were used to compare the incidence rate of LAM injury and urethral sphincter dysfunction in the corresponding groups. A conservative p value of 0.05 was considered a significant difference.
Table 1 shows the demographic information of the study population. The age and height were similar in primiparous groups, whereas body mass index (BMI) in women without SUI was higher. There was no difference in the age between the PC group and nulliparous group (p = 0.58), but significant difference between the PC and primiparous incontinent (PI) groups was observed (p < 0.001). Though women in the PC group were heavier compared to women in the nulliparous group, there was no significant difference in BMI (p = 0.18).
As per Table 2, 91.4% of the nulliparous women (32 cases) had normal muscles without visible defects, 5.7% (2 cases) with a minor, and 2.9% with major defect suggesting the existence of normal anatomic variations. The primiparous women were more likely to have a visible muscle defect, while the 33.3% (5 cases) of incontinent women had major defects and 31.4% (10 cases) of continent women had minor defects. Chi-square statistics from two-way contingency tables demonstrated that the distribution of none, minor, and major defects significantly differed between the PC group and nulliparous group, and between primiparous groups. There was no significant difference in the functional assessment in primiparous groups, but the larger levator hiatus in PC women in comparison with nulliparous women suggested the impact of birth (p = 0.05).
The similar urethral length in the three groups indicated that the urethral length was not changed after first vaginal delivery, and was not associated with the stress incontinent in primiparous women (Table 3). The urethral angle during rest and Valsalva status and the urethral mobility was similar in the primiparous group. Generally, the primiparous women have more urethral hypermobility compared with nulliparous women though without any statistical significance. The urethral sphincter dysfunction manifested as the widening of proximal urethra and funneling was observed in 80% of PI women, 21.2% of PC women, and 14.3% of nulliparous women, respectively.
In all the three groups, the location of the vesical neck was above the PCL during the status of rest (Table 3). But the vesical neck downward movement was more obvious in the PC group compared with the nulliparous group during Valsalva (p = 0.006). Moreover, the vesical neck descended more than 50% in the PI women during Valsalva compared with the PC women (p = 0.002).
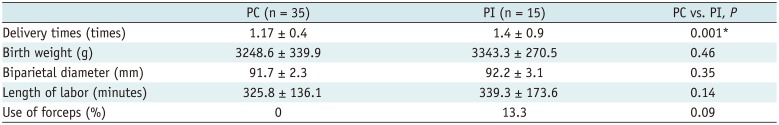
Obstetrical factors including pregnant times, birth weight, fetal biparietal diameter, length of labor, and forceps are listed in Table 4. Pregnant times in PI women were more than in PC women, and the difference was statistically significant (p = 0.001).
The present study demonstrated that there were substantial differences in both morphological changes of LAM and levator hiatus between nulliparous and PC women. Delancey and his co-workers first depicted LAM injury after a vaginal birth and suggested vaginal birth as a factor of levator ani injury (10). Our result was consistent with their findings, and we not only observed morphological changes of LAM but also found that the levator hiatus was markedly enlarged in postpartum women. We propose the following hypothesis for the occurrence of enlarged levator hiatus: Firstly, the existence of LAM function deficiency, especially in the puborectal and pubovisceral parts which enclosed the levator hiatus. Secondly, the intrinsic relaxation of anterior vaginal wall post vaginal delivery, which is the causes descending of pelvic organs such as bladder and uterus descending into the levator hiatus. Notably, there exists contradiction in the morphological and functional assessment of LAM in PI and PC groups. There were marked morphological changes in PI women, whereas no difference in iliococcygeal angle, levator plate, and levator hiatus was observed compared with the PC group. It seemed that the existence of minor or major LAM injury does not cause the functional difference.
The urethral mobility of primiparous women was seen to be markedly increased compared with the nulliparous women. The urethra lies on the supportive layer composed of the anterior vaginal wall endopelvic fascia, and the pubic part of LAM. Hence, when a woman has experienced a vaginal delivery, regardless of the occurrence of one or multiple situations like the intrinsic laxity of the anterior vaginal wall, the tear of the endopelvic fascia, and LAM injury, the urethra will manifest downward displacement and rotate around the inferior margin of the pubic symphysis. Our study suggested that there were substantial differences in vesical neck downward movement between nulliparous and PC groups, notably between primiparous groups. In contrast to the traditional opinion that urethral mobility is the primary contributing factor in SUI (91011), our study demonstrated that neither urethral angle during rest or Valsalva status nor urethral mobility differed between primiparous groups, indicating that these factors are not specific to primiparous women with SUI. Nevertheless, it was observed that the location of the vesical neck during straining and the vesical neck downward movement contributed more towards SUI.
Macura et al. (4) reported that SUI might be caused by the short urethra or small urethral muscle, but our study indicated that the urethral length was not decreased in PI women as proved by previous studies (8). It is hypothesized that the volume of connective tissue, vascular tissue and striated muscle, estrogen levels, and the valid length of the urethra decreases with increase in age. However, in our study, the short urethra was not noted in PI women. Descending and rotation of urethra as we suggested above contributes more towards SUI in PI women.
We demonstrated that urethral sphincter dysfunction was associated with de novo SUI in primiparous women. In our study, urethral sphincter dysfunction was defined by the widening of the proximal urethra at urethrovesical junction and funneling change at the vesical neck during straining on the sagittal plane. The funneling changes were observed in 80% of PI women and in 22.9% of PC women. Funneling can be treated as a valuable predictor for SUI in primiparous women. There was an unexpected observation of a low rate of identified funneling in nulliparous women without SUI symptoms. A longitudinal follow-up will be done to observe whether the PC women with funneling changes will develop SUI symptoms in the future and whether the nulliparous women with funneling changes will be prone towards the development of SUI after vaginal delivery.
There are significant challenges in studying the pelvic floor functional changes which are influenced by many factors such as obstetric factors, aging, and BMI in primiparous women. In our study, women without SUI were heavier, but not taller, resulting in greater BMI, which is contrary to the results provided by DeLancey et al. (8). So the role of increased weight or greater BMI in the development of SUI should be reserved. The lack of difference in most of the obstetrical factors such as birth weight, fetal biparietal diameter, length of labor, and forceps suggested that these obstetrical factors may be more strongly associated with pelvic organ prolapse than with SUI (1213). The significant difference in pregnant times in primiparous groups suggested the effect of multiple pregnant times on SUI.
Minor or major LAM injury, relative hypermobility of urethra, downward movement of vesical neck as well as urethral sphincter dysfunction occur in women after vaginal delivery, and all these contribute toward SUI. However, our aim is not to identify the most specific factor responsible for the development of SUI in primiparous women. We would rather treat all the parameters as a single global assessment standard because of their correlative and collaborative relationship with each other.
There are some limitations in our study. First, the size of the sample enrolled in the study was small, especially the age of the nulliparous group was in a smaller range. It was difficult to have women without the experience of pregnancy and delivery enrolled at the age of 30 years or above. Second, 6 months after vaginal delivery was used as a common point-in-time on rehabilitation of uterus and pelvic floor. The verification for the rehabilitation time point should be extended to 9 months and 12 months in future studies. Third, follow up studies on PC women and nulliparous women with funneling changes but without SUI symptoms were not performed.
In conclusion, de novo SUI after vaginal delivery was associated with major LAM injury, vesical neck downward movement as well as urethral sphincter dysfunction as observed on MRI. Vesical neck funneling on sagittal images can be treated as the main predictor. The intervention for the PI should focus on the elevation of vesical neck, rehabilitation of LAM as well as recovery of the urethral sphincter muscle.
Acknowledgments
We gratefully acknowledge the effort of Can Cui and Yanhong Wu for collecting and analysing the patients and Wen Shen for paper editing.
References
1. Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006; 107:1253–1260. PMID: 16738149.

2. Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Norwegian EPINCONT Study. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med. 2003; 348:900–907. PMID: 12621134.

3. Ashton-Miller JA, Howard D, DeLancey JO. The functional anatomy of the female pelvic floor and stress continence control system. Scand J Urol Nephrol Suppl. 2001; (207):1–7. discussion 106-125.
4. Macura KJ, Genadry RR, Bluemke DA. MR imaging of the female urethra and supporting ligaments in assessment of urinary incontinence: spectrum of abnormalities. Radiographics. 2006; 26:1135–1149. PMID: 16844938.

5. Tunn R, Goldammer K, Neymeyer J, Gauruder-Burmester A, Hamm B, Beyersdorff D. MRI morphology of the levator ani muscle, endopelvic fascia, and urethra in women with stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2006; 126:239–245. PMID: 16298035.

6. Peschers U, Schaer G, Anthuber C, Delancey JO, Schuessler B. Changes in vesical neck mobility following vaginal delivery. Obstet Gynecol. 1996; 88:1001–1006. PMID: 8942842.

7. Dietz HP, Bennett MJ. The effect of childbirth on pelvic organ mobility. Obstet Gynecol. 2003; 102:223–228. PMID: 12907092.

8. DeLancey JO, Miller JM, Kearney R, Howard D, Reddy P, Umek W, et al. Vaginal birth and de novo stress incontinence: relative contributions of urethral dysfunction and mobility. Obstet Gynecol. 2007; 110(2 Pt 1):354–362. PMID: 17666611.
9. Bergman A, McCarthy TA, Ballard CA, Yanai J. Role of the Q-tip test in evaluating stress urinary incontinence. J Reprod Med. 1987; 32:273–275. PMID: 3585870.
10. DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003; 101:46–53. PMID: 12517644.

11. Mostwin JL, Genadry R, Sanders R, Yang A. Anatomic goals in the correction of female stress urinary incontinence. J Endourol. 1996; 10:207–212. PMID: 8740379.

12. Patel DA, Xu X, Thomason AD, Ransom SB, Ivy JS, DeLancey JO. Childbirth and pelvic floor dysfunction: an epidemiologic approach to the assessment of prevention opportunities at delivery. Am J Obstet Gynecol. 2006; 195:23–28. PMID: 16579934.

13. Kearney R, Miller JM, Ashton-Miller JA, DeLancey JO. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006; 107:144–149. PMID: 16394052.

Fig. 1
T2-weighted MR images of different types of LAM injury (axial plane).
A. Score of “0.” Bilateral muscle parts have full connection to pubic bone and there is normal muscle bulk. B. Score of “1.” Slight avulsion in right side (arrow) is noted and less than half of unilateral muscle was lost. Left side is normal. C. Score of “2.” There is severe avulsion on left side with only minimal LAM connection to pubic bone (arrow). Right side is normal. D. Score of “3.” Normal muscle is seen on right side, whereas LAM in left side is completely detached from its insertion into pubis (arrow), and ipsilateral vaginal is seen to be collapsed with loss in its normal “H” shape (short arrow) and major injury of LAM was assessed in this patient. LAM = levator ani muscle, MR = magnetic resonance
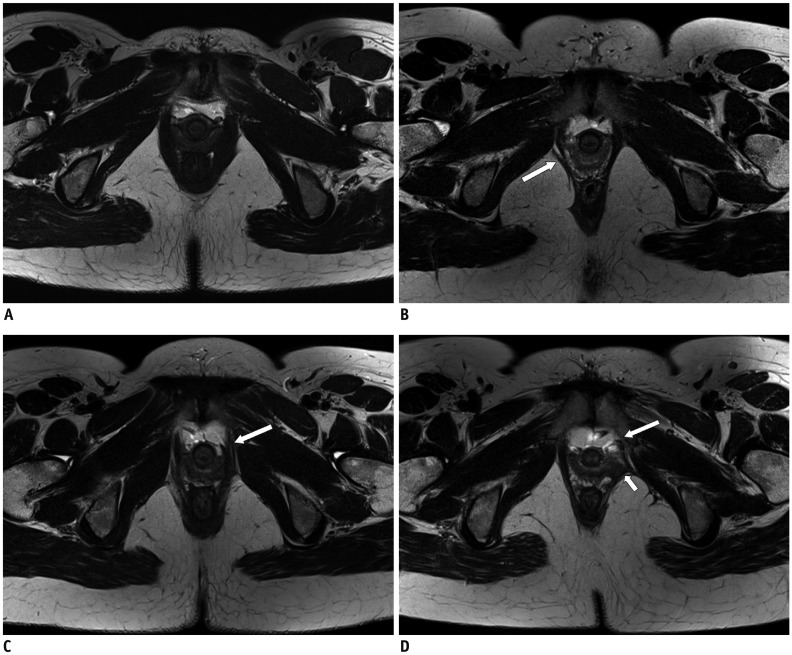
Fig. 2
Static and dynamic MR images illustrate measurement of LAM functional parameters.
Levator plate angles (black arrows) formed by levator plate and horizontal reference line during rest (A) and Valsalva status (B) on sagittal planes. Bilateral iliococcygeal angles (black arrows) measured as angles between iliococcygeal muscle and horizontal plane of pelvis during rest (C) and Valsalva status (D) on coronal planes. Levator hiatus measured as area demarcated anteriorly by inferior margin of pubis, posteriorly by inner aspect of puborectal muscle at anorectal junction (arrowheads), and bilaterally by pubovisceral muscles (arrows) during rest (E) and Valsalva (F) status on axial planes.
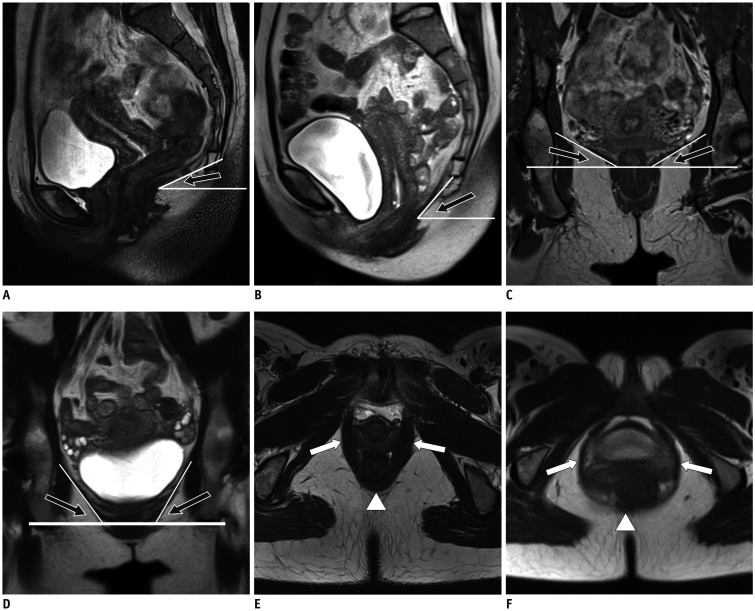
Fig. 3
Measurement of length of urethra, angle and mobility of urethra, and vesical neck movement.
Urethral angle was measured as angle between urethra and vertical axis, tangent line was used if urethra had slight rotation around pubic symphysis (A-C). Urethral mobility was defined as difference between value of α (B) and β (C) during rest and Valsalva status, respectively. If tip of urethral angle was protruding downwards, negative value was taken, whereas with reverse, positive value was taken. (D, E) Movement of vesical neck is defined as difference between its distances to PCL during rest and Valsalva status. Negative value was taken if vesical neck was located below PCL. PCL = pubococcygeal line
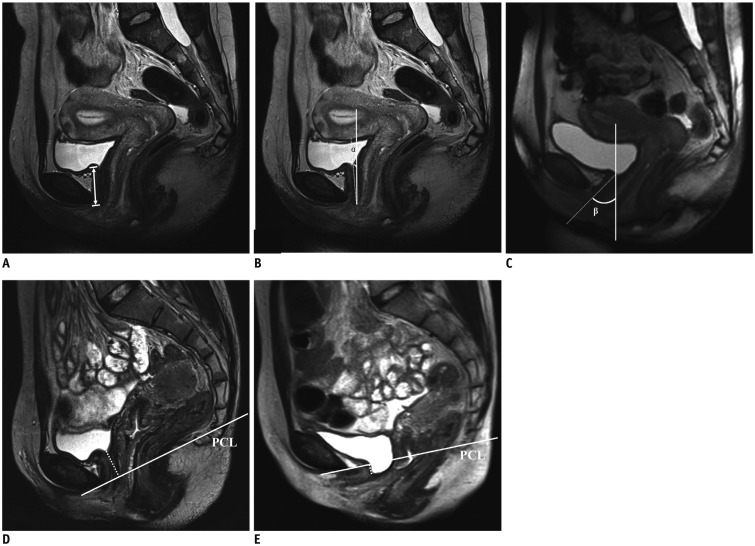
Fig. 4
Static and dynamic MR images show urethrovesical junction during rest and morphological changes during Valsalva on sagittal planes.
A, B. In primiparous woman without symptoms of SUI, hypermobility of urethra which descended and rotated into horizontal plane during Valsalva status is shown. But funneling of urethrovesical junction was invisible (white arrow). C, D. In primiparous woman with occasional symptoms of SUI, minimal funneling at urethrovesical junction is visible (white arrow) along with urethral hypermobility. E, F. In primiparous woman with frequent symptoms of SUI, widening of proximal urethra at vesical neck can be seen (white arrow) and funneling indicates weakness of proximal urethral sphincter. SUI = stress urinary incontinence
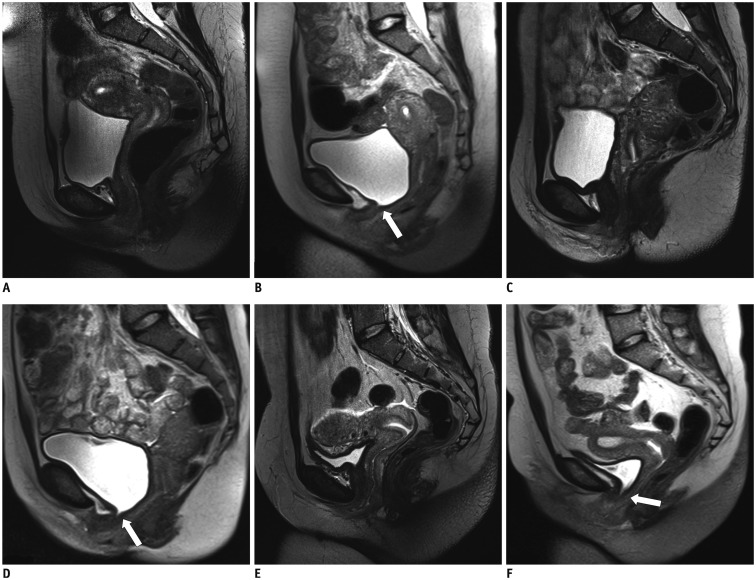
Table 1
Patients's Demographics
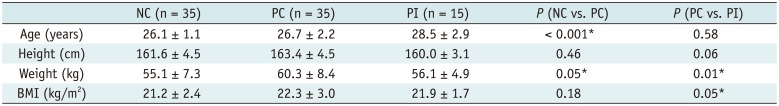
Table 2
Comparisons of LAM Injury and MR Imaging Parameters among Nulliparous Control Group, Primiparous Continent Group, and Primiparous Incontinent Groups
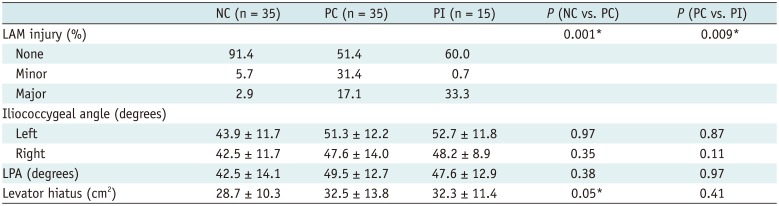
Table 3
Comparisons of Length, Mobility, and Function of Urethra among Nulliparous Control Group, Primiparous Continent Group, and Primiparous Incontinent Groups

Table 4
Compariosn of Obstetrical Variables between Primiparous Continent Group and Primiparous Incontinent Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download