INTRODUCTION
The ultrasonographic (US) feature of hypoechogenicity of a thyroid nodule is strongly associated with higher malignancy risk. Previous studies (
1234) consistently demonstrated that the malignancy risk of hypoechoic nodules was higher than that of isohyperechoic nodules. However, the US feature of hypoechogenicity is less specific for malignancy, and benign or malignant nodules may show various echogenicity of thyroid nodules. The degree of hypoechogenicity was reported to be related to the malignancy risk of thyroid nodules (
1) and has been clinically used for the malignancy risk stratification of thyroid nodules (
15). However, the histologic features in relation to the degree of nodule hypoechogenicity have not been investigated in thyroid nodules.
Previous studies (
67) have shown the association between the nodule hypoechogenicity and the histology feature of fibrosis. Chen et al. (
6) reported that the enlarged follicles showed highest echogenicity, papillary and follicular tumors showed intermediate echogenicity, and fibrosis showed the lowest echogenicity. However, these previous studies (
67) were based on the surgical specimen of thyroid nodules, which might make it difficult to directly correlate nodule echogenicity to histopathology features in large nodules showing heterogeneous echogenicity. Also, the relationship between nodule echogenicity and architectural pattern or cellularity of the nodule has not been investigated in the thyroid nodules. In our study, we directly compared the nodule echogenicity and various histologic features of nodules using the core needle biopsy (CNB) specimen which showed homogeneous echogenicity.
The purpose of this study was to investigate the association between the echogenicity and the histopathologic features of thyroid nodules, using the CNB specimens obtained in nodules with homogeneous echogenicity on US.
Go to :

MATERIALS AND METHODS
This study was approved by the Institutional Review Board. The informed consent for CNB procedure was obtained from all patients and the requirement for this informed consent was waived due to the retrospective nature of the study.
Study Population
From January 2014 to February 2015, a total of 199 thyroid nodules of 186 patients underwent CNB at a single institution.
Ninety-three nodules among 199 nodules were excluded for this study due to unsuitable conditions for the correlative analysis of nodule echogenicity and histologic features, which were mixed nodule echogenicity (n = 63), heavily calcified nodule (n = 16), predominantly cystic nodule (n = 11), nodule after radiofrequency ablation (n = 1), inadequate specimen (n = 1), and unavailable pathology slide (n = 1). Therefore, 106 nodules of 95 patients (76 women, 19 men; mean age 47.5 ± 12.9 years) were enrolled in this study and we randomly chose one of the two nodules in each patient who had two nodules, because the generalized estimating equation model failed to converge. Finally, we ended up analyzing 95 nodules of 95 patients.
US Examination and Image Analysis
We performed a high-resolution US scan using a 10–12 MHz linear-array transducer (AplioXG; Toshiba, Otawara, Japan). Two experienced radiologists (who had 19 years and 12 years of experience in performing thyroid US and interventional procedures, respectively) retrospectively reviewed these US images. They were unaware of the CNB results or final diagnoses and independently assessed the echogenicity of thyroid nodules. Consensus was used to determine the nodule echogenicity in the case of disagreement.
The echogenicity of thyroid nodules was categorized into 4 categories which were; markedly hypoechoic (hypoechoic or similar echogenicity relative to the sternocleidomastoid muscle), mildly hypoechoic (hypoechoic relative to the thyroid parenchyma, but not hypoechoic relative to the sternocleidomastoid muscle), isoechoic (similar echogenicity as that of the thyroid parenchyma), and hyperechoic (more echogenic relative to the thyroid parenchyma). We used the sternocleidomastoid muscle as the reference structure for determining the marked and mild hypoechogenicity because the hypoechogenicity of relatively thick sternocleidomastoid muscle is more constant and less sensitive to artefactual change of muscle echogenicity and gain adjustment than the anterior strap muscle. The definition of marked hypoechogenicity included the hypoechogenicity similar to the sternocleidomastoid muscle because the differentiation of more hypoechoic echogenicity than neck muscle and hypoechogenicity similar to neck muscle may be sometimes ambiguous and subjective.
Procedure and Histology Diagnosis of CNB
We selectively performed CNB in nodules with prior indeterminate fine-needle aspiration (FNA) results or suspicious US features for malignancy and follicular neoplasm, and also when there was a concern about inadequate FNA result during the FNA procedure (
8910). An experienced thyroid radiologist, with 10 years of experience in CNB procedure, performed the CNB using a disposable 18-gauge, single- or double-action spring-activated needle (TSK Acecut or Stericut; Create Medic, Yokohama, Japan), as described elsewhere (
89). He positioned the needle notch of CNB to cut some portion of normal parenchyma (about 2 mm in length) at the nodule margin, if technically feasible; the number of CNB biopsies was 1 or 2. After patients underwent biopsy, we immediately compressed the biopsy site and observed them during manual self-compression of the biopsy site for 20–30 minutes. The interpretation of CNB results were routinely reported by a six-tier pathology reporting system by an experienced endocrine pathologist, and the 6 categories of CNB histology diagnoses were non-diagnostic, benign, indeterminate, follicular neoplasm or suspicious for a follicular neoplasm (FN/SFN), suspicious for malignancy, and malignant (
11).
Analysis of CNB Histology Features
Each CNB specimen was retrospectively reviewed and interpreted by an experienced endocrine pathologist in 95 thyroid nodules. The pathologist was unaware of the reported CNB diagnosis, final diagnosis, and US features of thyroid nodules. The interpreter evaluated the histology features of CNB specimens by 5 categories; the degree of fibrosis, the amount of lymphoid infiltration, microfollicular pattern, the uniform follicular pattern, and the degree of cellularity based on the major histopathologic characteristics of non-neoplastic and neoplastic thyroid nodules (
12).
The presence of fibrosis or lymphoid infiltration was determined when the proportion of fibrosis or lymphoid infiltration was more than 30% in the given CNB specimen.
The microfollicular pattern was defined when the follicular lesion was mostly composed of microfollicles more than 90% of the follicular lesion, and the uniform follicular pattern was defined when more than 90% of follicles showed minimal size variation in the specimen. The high cellularity was determined when the cellularity of a nodule was conspicuously higher than normal thyroid follicles.
The microfollicular or uniform follicular pattern was determined by the evaluation of only follicular lesion in the given specimen. The cellularity of the lesion was determined by evaluation of the overall cellularity of any pathologic lesion in the specimen.
Data Analysis and Statistics
We performed an interrater reliability analysis using the weighted Kappa statistic, to determine consistency in raters when determining the nodule echogenicity. The chi-square test or Fisher's exact test was used to compare the frequency of each histology feature and the frequency of each CNB diagnostic result among 3 categories of the nodule echogenicity. The Cochran-Armitage trend test was used to investigate the trends between the nodule echogenicity and the frequencies of histology features and benign or malignant CNB diagnostic results. Post-hoc pairwise analysis was performed using a Bonferroni correction. Multivariable multinomial logistic regression analysis was used to assess independent associations between nodule echogenicity and histology features. The results are presented as odds ratio (OR) with 95% confidence interval (CI). Statistical analysis was performed with SAS v9.2 (SAS Institute Inc., Cary, NC, USA) and a significant difference was defined as a p value of < 0.05 in all statistics except for the post-hoc pairwise analysis using a Bonferroni correction in which a p value of < 0.0167 was used for the significant difference.
Go to :

RESULTS
Demographic Data
The maximal size of the nodules ranged from 5 mm to 70 mm (mean size, 14.0 ± 10.9 mm; median size, 11.0 mm). Of the 95 thyroid nodules, there were 28 (29.5%) isoechoic nodules, 37 (38.9%) mildly hypoechoic nodules, 30 (31.6%) markedly hypoechoic nodules, and no hyperechoic nodule. The CNB histology diagnoses of 95 nodules were 1 (1.1%) non-diagnostic, 51 (53.7%) benign, 12 (12.6%) indeterminate, 5 (5.3%) FN/SFN, and 26 (27.4%) malignant nodules. Regarding the nodule echogenicity, the interrater agreement for the raters was found to be weighted Kappa = 0.887 (p < 0.001), 95% CI (0.815, 0.958) (almost perfect agreement). There was no major complication although one patient (0.5%, 1/186) developed a minor complication of intrathyroidal hemorrhage with edema after CNB during the study period. The parenchymal hemorrhage and edema was resolved following compression for approximately one hour.
Relationship of Nodule Echogenicity and Histologic Features in 95 Thyroid Nodules
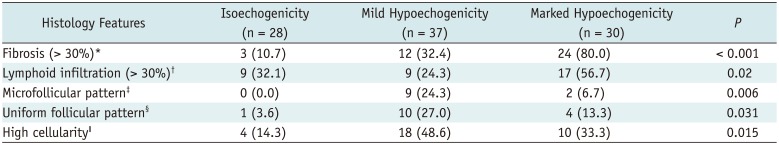
Table 1 shows the frequency of 5 histology features in each nodule echogenicity of thyroid nodules. The frequency of 5 histology features had a significant difference among the 3 categories of nodule echogenicity (
p ≤ 0.02) (
Figs. 1,
2,
3,
4). There was a trend of increasing frequency of fibrosis (> 30%) with decreasing nodule echogenicity (
p < 0.001) and a marginal trend of increasing frequency of lymphoid infiltration (> 30%) with decreasing nodule echogenicity (
p = 0.048). There was no trend in the frequency of other histology features with change nodule echogenicity (
p ≥ 0.141).
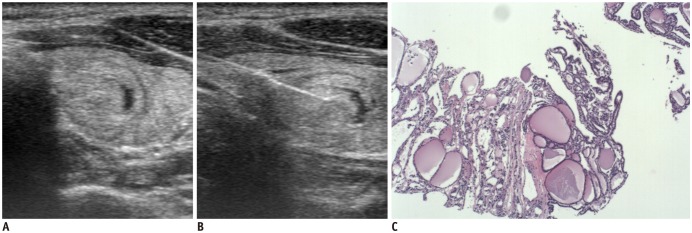 | Fig. 1
Ultrasonography and CNB histology features of nodular hyperplasia diagnosed on CNB.
Transverse ultrasonography image shows ovoid isoechoic and predominantly solid nodule (A), and needle notch of CNB is positioned within nodule and perinodular normal thyroid tissue (B). CNB histology (C) shows variable sized follicular lesion without any of fibrosis, microfollicular pattern, and high cellularity (haematoxylin-eosin staining, × 40). CNB = core needle biopsy

|
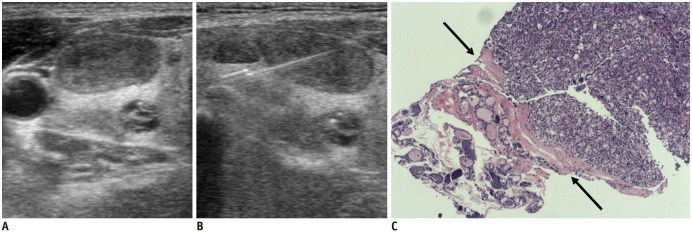 | Fig. 2
Ultrasonography and CNB histology features of suspicious follicular neoplasm diagnosed on CNB.
Transverse ultrasonography image shows ovoid mildly hypoechoic solid nodule (A), and needle notch of CNB is positioned within nodule and perinodular normal thyroid tissue (B). CNB histology (C) shows follicular lesion which shows uniform and microfollicular architectural patterns, high cellularity, and no fibrosis, and fibrous capsule (arrows) is seen between follicular lesion and normal thyroid tissue (haematoxylin-eosin staining, × 40).

|
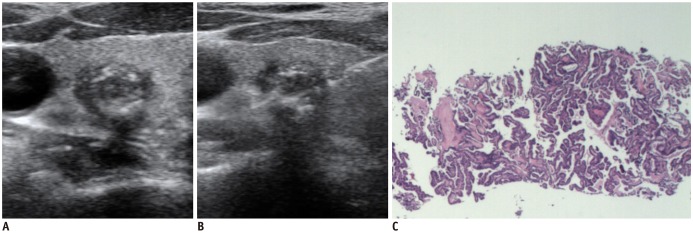 | Fig. 3
Ultrasonography and CNB histology features of conventional papillary carcinoma diagnosed on CNB.
Transverse ultrasonography image shows round markedly hypoechoic solid nodule with macrocalcifications and spiculated margin (A), and needle notch of CNB is positioned within center of nodule and perinodular normal thyroid tissue (B). CNB histology (C) shows high cellular tumor cells and no histology feature of fibrosis, uniform follicular pattern, and microfollicular pattern (haematoxylin-eosin staining, × 40).

|
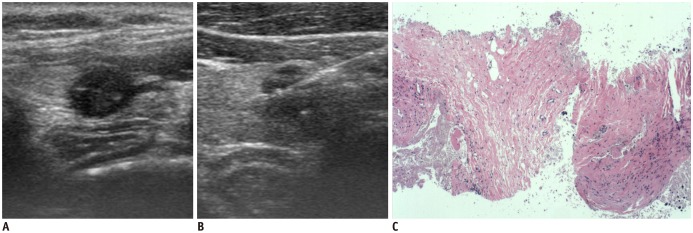 | Fig. 4
Ultrasonography and CNB histology features of fibrotic nodule diagnosed as non-diagnostic on CNB.
Transverse ultrasonography image shows round markedly hypoechoic solid nodule (A), and needle notch of CNB is positioned within nodule (B). CNB histology (C) shows only fibrosis and no follicular lesion (haematoxylin-eosin staining, × 40).

|
Table 1
Frequency of Histologic Features in Each Nodule Echogenicity of 95 Thyroid Nodules
|
Histology Features |
Isoechogenicity (n = 28) |
Mild Hypoechogenicity (n = 37) |
Marked Hypoechogenicity (n = 30) |
P
|
|
Fibrosis (> 30%)*
|
3 (10.7) |
12 (32.4) |
24 (80.0) |
< 0.001 |
|
Lymphoid infiltration (> 30%)†
|
9 (32.1) |
9 (24.3) |
17 (56.7) |
0.02 |
|
Microfollicular pattern‡
|
0 (0.0) |
9 (24.3) |
2 (6.7) |
0.006 |
|
Uniform follicular pattern§
|
1 (3.6) |
10 (27.0) |
4 (13.3) |
0.031 |
|
High cellularityǁ
|
4 (14.3) |
18 (48.6) |
10 (33.3) |
0.015 |

Post-hoc analysis showed that the frequency of fibrosis (> 30%) was significantly different between isoechoic nodules and markedly hypoechoic nodules, and between mildly hypoechoic and markedly hypoechoic nodules (p < 0.001, respectively). There was a tendency of higher frequency of fibrosis (> 30%) in mildly hypoechoic nodules than in isoechoic nodules, but statistically insignificant (p = 0.040). There was a difference in lymphoid infiltration (> 30%) only between mildly hypoechoic and markedly hypoechoic nodules (p = 0.007). The frequency of microfollicular pattern and high cellularity was significantly higher in mildly hypoechoic nodules than in isoechoic nodules (p = 0.008 and p = 0.004, respectively), and the frequency of uniform follicular pattern was higher in mildly hypoechoic nodules (p = 0.018). There was no significant difference in histology features of microfollicular pattern, uniform follicular pattern, and high cellularity between mildly and markedly hypoechoic nodules and between isoechoic and markedly hypoechoic nodules (p ≥ 0.09). The histology features of microfollicular pattern and uniform follicular pattern were predominantly found in mildly hypoechoic nodules (81.8% and 66.7% of each histology feature, respectively) and mostly in mildly or markedly hypoechoic nodules (100% and 93.3% of each histology feature, respectively). All of 11 nodules with microfollicular pattern showed histology features of uniform follicular pattern and high cellularity. The microfollicular pattern was found in 11 (73.3%) and high cellularity in 14 (93.3%) of 15 nodules with uniform follicular pattern. The histology feature of fibrosis (> 30%) or high cellularity was found in 29 (78.3%) of 37 nodules with mild hypoechogenicity and in 26 (86.7%) of 30 nodules with marked hypoechogenicity.
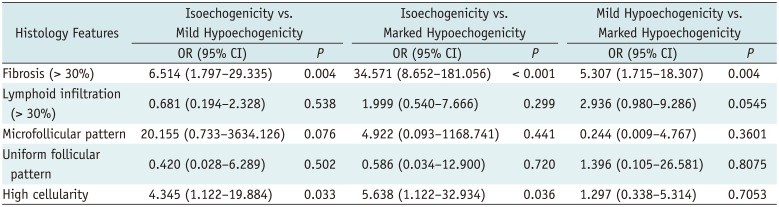
Table 2 shows the independent associations between histology features and nodule echogenicity by multivariable multinomial logistic regression analysis in all nodules. The multivariable multinomial logistic regression analysis demonstrated that the histology features significantly associated with mildly hypoechoic nodules were fibrosis (OR = 6.514) and high cellularity (OR = 4.345) compared with isoechoic nodules as reference (
p = 0.004 and 0.033, respectively), and the histology features significantly associated with markedly hypoechoic nodules were fibrosis (> 30%) (OR = 34.571) and high cellularity (OR = 5.638) compared with isoechoic nodules as reference (
p < 0.001 and
p = 0.036, respectively). Only fibrosis (> 30%) was significantly associated with markedly hypoechoic nodules compared with mildly hypoechoic nodules as reference (OR = 5.307,
p = 0.004).
Table 2
Multivariable Multinomial Logistic Regression Analysis of Nodule Echogenicity and Histologic Features in 95 Thyroid Nodules
|
Histology Features |
Isoechogenicity vs. Mild Hypoechogenicity |
Isoechogenicity vs. Marked Hypoechogenicity |
Mild Hypoechogenicity vs. Marked Hypoechogenicity |
|
OR (95% CI) |
P
|
OR (95% CI) |
P
|
OR (95% CI) |
P
|
|
Fibrosis (> 30%) |
6.514 (1.797–29.335) |
0.004 |
34.571 (8.652–181.056) |
< 0.001 |
5.307 (1.715–18.307) |
0.004 |
|
Lymphoid infiltration (> 30%) |
0.681 (0.194–2.328) |
0.538 |
1.999 (0.540–7.666) |
0.299 |
2.936 (0.980–9.286) |
0.0545 |
|
Microfollicular pattern |
20.155 (0.733–3634.126) |
0.076 |
4.922 (0.093–1168.741) |
0.441 |
0.244 (0.009–4.767) |
0.3601 |
|
Uniform follicular pattern |
0.420 (0.028–6.289) |
0.502 |
0.586 (0.034–12.900) |
0.720 |
1.396 (0.105–26.581) |
0.8075 |
|
High cellularity |
4.345 (1.122–19.884) |
0.033 |
5.638 (1.122–32.934) |
0.036 |
1.297 (0.338–5.314) |
0.7053 |

Relationship of Nodule Echogenicity and Histologic Features in 56 Thyroid Nodules without Fibrosis > 30%
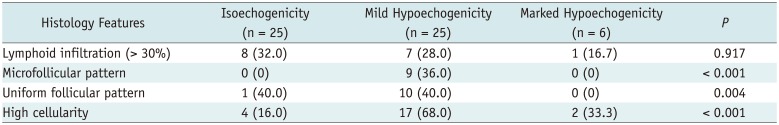
In the 56 nodules without fibrosis > 30%, the frequency of three histology features of microfollicular pattern, uniform follicular pattern, and high cellularity showed a significant difference among 3 categories of nodule echogenicity (
p = 0.001, 0.004, and 0.001, respectively) (
Table 3). Post-hoc analysis showed that the frequency of microfollicular pattern, uniform follicular pattern, and high cellularity were significantly higher in mildly hypoechoic nodules than in isoechoic nodules (
p = 0.002, 0.006, and 0.001, respectively). There was no significant difference in the frequency of each histology feature between isoechoic nodules and markedly hypoechoic nodules or between mildly hypoechoic and markedly hypoechoic nodules (
p ≥ 0.141). Although all nodules with microfollicular pattern showed mild hypoechogenicity, nodules without microfollicular pattern showed various nodule echogenicity which was isoechogenicity in 25 (53.2%), mild hypoechogenicity in 16 (34%), and marked hypoechogenicity in 6 (12.8%) of 47 nodules.
Table 3
Frequency of Histologic Features in Each Nodule Echogenicity of 56 Thyroid Nodules without Fibrosis > 30%
|
Histology Features |
Isoechogenicity (n = 25) |
Mild Hypoechogenicity (n = 25) |
Marked Hypoechogenicity (n = 6) |
P
|
|
Lymphoid infiltration (> 30%) |
8 (32.0) |
7 (28.0) |
1 (16.7) |
0.917 |
|
Microfollicular pattern |
0 (0) |
9 (36.0) |
0 (0) |
< 0.001 |
|
Uniform follicular pattern |
1 (40.0) |
10 (40.0) |
0 (0) |
0.004 |
|
High cellularity |
4 (16.0) |
17 (68.0) |
2 (33.3) |
< 0.001 |

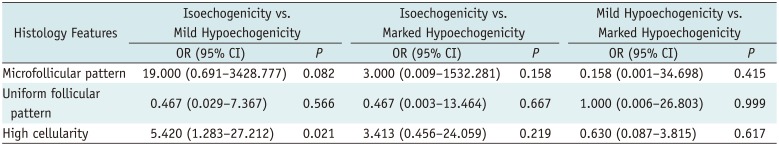
Table 4 shows the independent associations between histology features and nodule echogenicity by multivariable multinomial logistic regression analysis in 56 nodules without fibrosis > 30%. The multivariable multinomial logistic regression analysis demonstrated that the mildly hypoechoic nodules showed significantly higher frequency of high cellularity (OR = 5.420) compared to isoechoic nodules as reference (
p = 0.021). There was no significant independent association of any histology feature between isoechoic nodules and markedly hypoechoic nodules or between mildly hypoechoic and markedly hypoechoic nodules (
p ≥ 0.158).
Table 4
Multinomial Logistic Regression Analysis of Nodule Echogenicity and Histologic Features in 56 Thyroid Nodules without Fibrosis > 30%
|
Histology Features |
Isoechogenicity vs. Mild Hypoechogenicity |
Isoechogenicity vs. Marked Hypoechogenicity |
Mild Hypoechogenicity vs. Marked Hypoechogenicity |
|
OR (95% CI) |
P
|
OR (95% CI) |
P
|
OR (95% CI) |
P
|
|
Microfollicular pattern |
19.000 (0.691–3428.777) |
0.082 |
3.000 (0.009–1532.281) |
0.158 |
0.158 (0.001–34.698) |
0.415 |
|
Uniform follicular pattern |
0.467 (0.029–7.367) |
0.566 |
0.467 (0.003–13.464) |
0.667 |
1.000 (0.006–26.803) |
0.999 |
|
High cellularity |
5.420 (1.283–27.212) |
0.021 |
3.413 (0.456–24.059) |
0.219 |
0.630 (0.087–3.815) |
0.617 |

Relationship of CNB Diagnosis and Nodule Echogenicity
Table 5 shows the distribution of 6 CNB diagnostic results among 3 categories of nodule echogenicity. The frequencies of benign or malignant CNB diagnostic results were significantly different among 3 categories of nodule echogenicity (
p < 0.001, respectively).
Table 5
CNB Diagnostic Results and Echogenicity of Thyroid Nodules
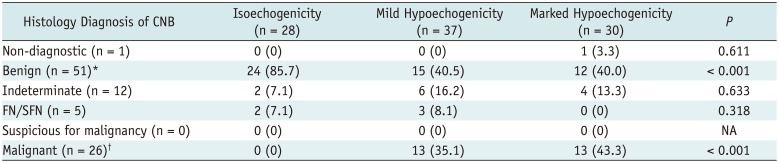
|
Histology Diagnosis of CNB |
Isoechogenicity (n = 28) |
Mild Hypoechogenicity (n = 37) |
Marked Hypoechogenicity (n = 30) |
P
|
|
Non-diagnostic (n = 1) |
0 (0) |
0 (0) |
1 (3.3) |
0.611 |
|
Benign (n = 51)*
|
24 (85.7) |
15 (40.5) |
12 (40.0) |
< 0.001 |
|
Indeterminate (n = 12) |
2 (7.1) |
6 (16.2) |
4 (13.3) |
0.633 |
|
FN/SFN (n = 5) |
2 (7.1) |
3 (8.1) |
0 (0) |
0.318 |
|
Suspicious for malignancy (n = 0) |
0 (0) |
0 (0) |
0 (0) |
NA |
|
Malignant (n = 26)†
|
0 (0) |
13 (35.1) |
13 (43.3) |
< 0.001 |

There was a trend of increasing frequency of benign diagnosis with increasing nodule echogenicity (p = 0.001) and a trend of increasing frequency of malignant diagnosis with decreasing nodule echogenicity (p = 0.001). Among 3 categories of nodule echogenicity, the frequency of benign or malignant diagnosis was significantly different between isoechoic nodules and mildly or markedly hypoechoic nodules (p < 0.001, respectively). There was no significant difference in the frequency of benign or malignant diagnosis between mildly hypoechoic and markedly hypoechoic nodules (p = 0.964, p = 0.493, respectively).
Go to :

DISCUSSION
Our study demonstrated that the fibrosis (> 30%) and high cellularity were independently associated with mild or marked hypoechogenicity as compared to isoechogenicity, and only fibrosis (> 30%) was independently associated with marked hypoechogenicity as compared to mild hypoechogenicity in thyroid nodules. There was a trend of increasing frequency of fibrosis (> 30%) as the nodule echogenicity decreased. The frequency of microfollicular pattern and hypercellularity was significantly higher in mildly hypoechoic nodules than in isoechoic nodules. Our study showed that the nodule echogenicity can predict the histologic features of nodules, which may provide helpful information for the management of thyroid nodules.
The tissue echogenicity is related to the acoustic reflection caused by variations in the acoustic impedance (
13). The normal thyroid gland showed homogeneous bright echogenicity which are related to multiple acoustic interfaces of normal thyroid tissue composed of follicles (follicular cells and colloid) and stroma. The hypoechogenicity of thyroid nodules composed of dense fibrosis or hypercellular lesions may be explained by the decreased number of acoustic interfaces and less acoustic reflection compared to normal thyroid tissue.
Our study shows that fibrosis (> 30%) is strongly associated with marked hypoechogenicity of nodules, same as previous studies results (
67). Our study also shows that the histologic feature of high cellularity is associated with the marked hypoechogenicity of nodules, which explains why focal thyroiditis with severe lymphocytic infiltration, high cellular follicular lesion, and high cellular malignant tumors may show marked hypoechogenicity. However, the histology feature of fibrosis (> 30%) played a dominant role for marked hypoechogenicity, because the OR of fibrosis (> 30%) for marked hypoechogenicity was approximately 6 times higher than the OR of high cellularity with a reference of isoechogenicity, and only fibrosis (> 30%) was independently associated with marked hypoechogenicity compared with mild hypoechogenicity in thyroid nodules.
All nodules with microfollicular pattern showed uniform follicular pattern, high cellularity, and hypoechogenicity (mild or marked). In these nodules, we found mild hypoechogenicity in 81.8% of all nodules and in 100% of subgroup nodules without fibrosis (> 30%). In 5 nodules diagnosed as FN/SFN by CNB, 3 nodules with microfollicular pattern showed mild hypoechogenicity and 2 nodules without microfollicular pattern showed isoechogenicity. These results suggested that the echogenicity of follicular lesion may be related to the architectural pattern as well as fibrosis or overall cellularity of a nodule. Our study suggests that the follicular lesions with microfollicular pattern mostly showed hypoechogenicity and follicular lesions with normofollicular or macrofollicular pattern may show variable echogenicity. The histology features of microfollicular pattern, uniform follicular pattern, and high cellularity are the characteristic histology features of follicular neoplasm (
111214) and the follicular neoplasm with prominent microfollicular pattern may mostly show mild hypoechogenicity.
Benign or malignant nodules showed variable echogenicity according to the mixed composition of histology features which mainly included fibrosis and overall cellularity. The histology feature of fibrosis is frequently found in papillary thyroid carcinomas (PTC) (
7) and dense fibrosis is strongly associated with PTC (
15). However, dense fibrosis can be found in degenerating benign thyroid nodules (
161718). The US-pathologic correlation of malignant-looking degenerating benign nodules showed that the central hypoechoic area was primarily composed of fibrosis and hemorrhage and that the isoechoic rim was composed of follicular cells (
18). The US features of degenerating or mummified nodules and collapsing cystic benign nodules frequently mimic malignant US features (
161718). The US features of ovoid-to-round shape, ill-defined margin, inner isoechoic rim, and low-echoic halo was useful for differentiating collapsing cystic nodules from malignancy (
16), and the US features of regular eggshell calcifications, peripheral hypoechoic or hypoechoic rim, posterior shadowing, absence of intranodular vascularization, and nodule shrinkage over time were useful findings for the diagnosis of mummified nodules (
17). The understanding of the US features and pathologic features of degenerating benign nodules may reduce unnecessary repeated biopsy or diagnostic surgery.
The histology feature of high cellularity is mainly found in follicular patterned lesions with microfollicular pattern and non-follicular patterned neoplasms with high cellular neoplastic cells.
The thyroid nodules with high cellularity may include hypercellular follicular patterned nodules (nodular hyperplasia, follicular neoplasm, and follicular variant PTC), non-follicular patterned neoplasms (conventional PTC and other malignant tumors), and nodular inflammatory lesion (focal thyroiditis). The histology features of fibrosis and high cellularity which are closely related to the hypoechogenicity of nodules are more frequently found in PTC (most common malignancy) than in nodular hyperplasia (most common benign nodules), which may explain the reason why the US feature of hypoechogenicity is more common in malignant nodules and is predictive of malignancy in thyroid nodules (
1234). Although it is difficult to predict the dominant histology feature of hypoechoic nodules on gray-scale US, color Doppler US may be helpful for differentiation of dense fibrosis and hypercellular lesion, because hypercellular nodules without dense fibrosis may show intranodular vascularity.
Our study is the first to investigate the relationship between the echogenicity and histologic features of thyroid nodules using the CNB specimen. Our study suggests that FNA or CNB should be performed at each component showing different echogenicity in nodules with heterogeneous echogenicity because the histologic features are different according to the nodule echogenicity. The histologic diagnosis of follicular neoplasm were found mostly in nodules with isoechogenicity and mild hypoechogenicity, which suggests that the CNB specimen should include the nodule margin to determine the presence of nodular capsule in these nodules. Meanwhile, the sufficient tissue sampling of the nodule itself may be more important for the accurate FNA or CNB diagnosis in nodules with marked hypoechogenicity because the histology feature of fibrosis were found in the majority of these nodules. Cautious follow-up may be required in mildly or markedly hypoechoic nodules even though the initial FNA result was benign because the frequency of fibrosis, microfollicular follicular pattern, and high cellularity which increase the risk of thyroid neoplasm is high in these nodules.
There are several limitations to this study. First, the sample size was small and uncommon neoplasms were not included in this study. Secondly, we could not evaluate the relationship between the nodule echogenicity and the mild degree of histology features such as focal microfollicular pattern or slightly increased cellularity. We used the obvious histology criteria to minimize the possible interpretation bias. Thirdly, we did not assess the histology features of nodules with mixed echogenicity. Fourth, the relationship between nodule echogenicity and CNB diagnostic category can not be generalized because many nodules unsuitable for this study were excluded from the consecutive data. Fifth, we did not assess the intranodular vascularity of thyroid nodules.
In conclusion, the histology features of fibrosis (> 30%) and high cellularity are independently associated with mild or marked hypoechogenicity of nodules, and fibrosis (> 30%) is strongly associated with marked hypoechogenicity of nodules.
The knowledge of the relationship of echogenicity and histopathology of thyroid nodules may provide helpful information for the US-based prediction of the histopathology of nodules, biopsy procedure, and follow-up of nodules, which could improve management of patients with thyroid nodules.
Go to :














 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download