Abstract
The Korean Society of Radiology and the National Evidence-based Healthcare Collaborating Agency developed guidelines for primary imaging tests and appropriate biopsy methods for thyroid nodules. These guidelines were developed using an adaptation process by collaboration between the development committee and the working group. The development committee, composed of research methodology experts, established the overall plan and provided support about methodological strategies. The working group, composed of radiologist experts in thyroid imaging, wrote the proposals. The guidelines recommend neck ultrasound (US) as a first-line imaging modality for the diagnosis of thyroid nodules in patients with suspected nodules, and US-guided fine-needle aspiration as a primary method for histologic examination of thyroid nodules.
Thyroid nodules are common in clinical practice. They are found in 4–8% of the general population via palpation, in 19–67% of patients via ultrasound (US), and in 50% of autopsy specimens (123456). Malignancies occur in 5–15% of thyroid nodules (378), and the incidence of thyroid cancer continues to increase worldwide due to various factors including genetic and environmental factors, development of medical devices, and the widespread implementation of healthcare programs. In Korea, thyroid cancer was the most common cancer in adults in 2013, being the most prevalent cancer in females and sixth most prevalent in males (9).
Various imaging techniques and procedures have been developed for the differential diagnosis of thyroid nodules. Imaging techniques include US, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). Procedures including palpation-guided fine-needle aspiration (FNA), US-guided FNA, and US-guided core needle biopsy (CNB) allow confirmation of nodules (1011). The need for standardized evidence-based clinical guidelines for the management of thyroid nodules has been proposed, and the Korean Society of Radiology (KSR) and the National Evidence-based Healthcare Collaborating Agency (NECA) organized a development committee and working group to develop such guidelines (12).
The guideline development process involved collaboration between NECA and KSR. NECA is a national agency that provides evidence-based information about medical devices, medicines, and health technology obtained through objective and reliable analyses. The development committee included medical imaging experts, research methodology specialists, and clinical guideline specialists who support the overall planning and research methodology. They published a methodology for the guideline adaptation process in imaging diagnosis (12). The working group included expert members of the Korean Society of Thyroid Radiology (KSThR). KSThR is an organization composed of thyroid radiologists in Korea that is primarily involved in the diagnosis and nonsurgical treatment of thyroid nodules. KSThR has published several sets of guidelines for thyroid imaging (1113), biopsy (1014), and radiofrequency ablation (15). The working group participated in the guideline development process and wrote recommendations (12).
The guideline development process employed an adaptation methodology for Korean clinical imaging guidelines (K-CIG) established by the development committee (12).
The Key Questions selected by the working group were reviewed by the development committee and a consensus group composed of clinical experts. Two final Key Questions were identified:
1) What is the primary imaging test for diagnosis in patients with suspected thyroid nodules?
2) What is the appropriate biopsy method for thyroid nodules?
A systematic search for guidelines was performed using international databases published up to July 2015, including Ovid-MEDLINE, Ovid-EMBASE, National Guideline Clearinghouse, Guideline International Network, and major domestic databases including KoreaMed, KMBASE, Korean Medical Guidelines and Information, and Korean Guideline Clearinghouse. The websites of major academic societies and institutions were also searched.
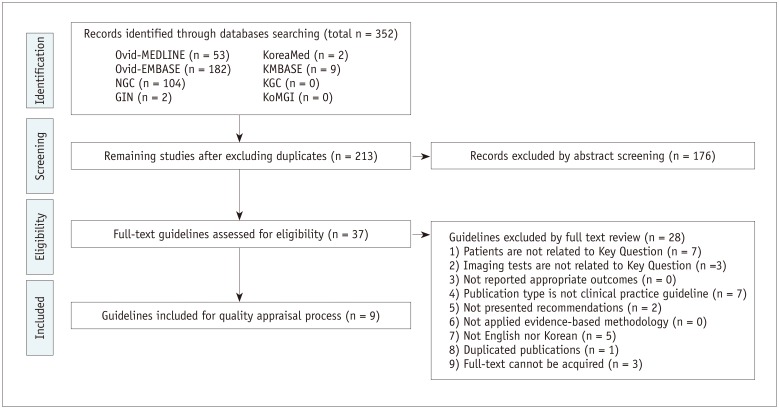
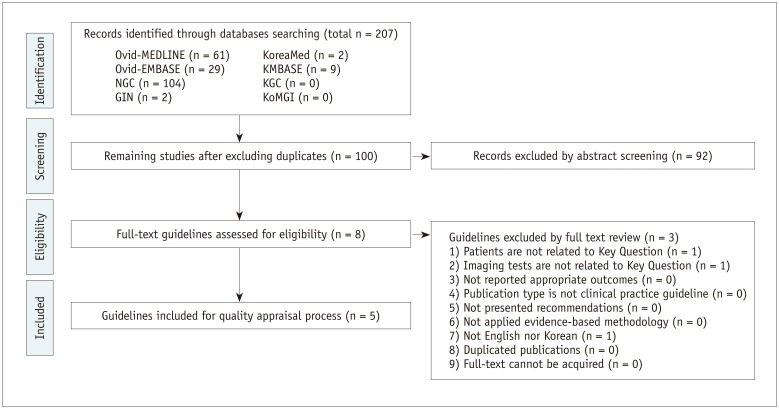
A total of 352 and 207 studies addressed Key Questions 1 and 2, respectively. Guideline selection was performed independently based on predefined inclusion/exclusion criteria. After excluding duplicates, screening titles and abstracts, and reading the full texts of the remaining articles, 9 and 5 articles, respectively, were selected (Figs. 1, 2).
The selected guidelines underwent a quality appraisal process using the Korean Appraisal of Guidelines for Research and Evaluation II tool developed by the guideline development committee. Guidelines with a score of less than 50 in the “Rigour of Development” domain were not recommended by the development committee. An exception was the “ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations” (13), which was selected despite its low score because it was established by domestic thyroid-imaging experts. After this process, five guidelines each were selected for Key Questions 1 and 2 (Tables 1, 2).
The working group members reviewed evidence literatures that supported the recommendations of the selected guidelines. Grading the level of evidence of each literatures was performed according to the evidence level criteria of the K-CIG (12). After discussion and comparison of recommendations and their evidence literatures of selected guidelines, draft recommendations were prepared by the working group.
The draft written by working group was reviewed and discussed by the development committee. Then, together, both groups came to a consensus on the recommendations grade and the evidence level, according to the criteria of the K-CIG (12).
The Delphi method was used for formal consensus. The consensus group was composed of clinical imaging experts, clinical imaging guidelines-related academic societies (end-users), and research methodology experts. The agreement level for each recommendation, the grade of each recommendation, and the evidence level were rated from strongly disagree (level 1) to strongly agree (level 9). After two rounds of assessment, a consensus was reached. The average degree of agreement was 7.80 (standard deviation: 1.25) for recommendation 1 and 7.60 (standard deviation: 1.19) for recommendation 2.
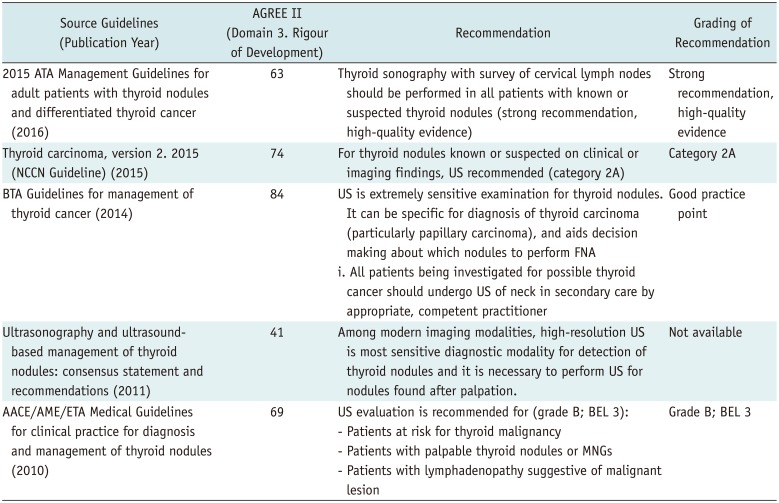
After reviewing the guidelines for diagnosis and treatment of thyroid nodules, final five guidelines were selected (1316171819). In all five guidelines, neck US is recommended for diagnosis when a thyroid nodule is suspected. US is a highly sensitive diagnostic method for thyroid nodules and can be used to diagnose nodules and determine the need for FNA tests.
According to US and the US-based management of thyroid nodules: consensus statement and recommendations, high-resolution neck US is the most sensitive method for detecting thyroid nodules, and a neck US examination is recommended for diagnosis of suspected thyroid nodules (13). US is used to determine the size and morphologic features of the nodule, diagnose nodal involvement, and determine the need and possibility of US-guided FNA.
According to the American Thyroid Association (ATA) Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer, US of the thyroid gland and cervical lymph nodes is recommended in patients with suspected thyroid nodule or with incidental thyroid nodules detected via CT, MRI, or PET, for the diagnosis of thyroid nodule (17). It is recommended to confirm via neck US whether the lesion causing the symptom is related to the thyroid nodule; analyze the location, size, and morphological characteristics of the nodule; and ascertain whether neck lymph node metastasis has occurred (202122).
The British Thyroid Association (BTA) Guidelines for the Management of Thyroid Cancer define neck US as a sensitive technique for the diagnosis of thyroid nodules and recommend neck US for differential diagnosis of papillary thyroid cancer (18). US is useful for determining the US-guided FNA test according to the morphologic findings of the nodule and for increasing the diagnosis rate (2324).
According to the American Association of Clinical Endocrinologists (AACE), Associazione Medici Endocrinologi (AME), and European Thyroid Association (ETA) Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules, neck US is the most useful test for the detection and diagnosis of thyroid nodule, and for the determining changes in the thyroid parenchyma (16). These guidelines recommend US if a nodule needs to be touched or if there is suspicion of thyroidopathy (25), as well as to confirm thyroid pathology in the presence of palpable neck lymph nodes, because cervical lymph node metastasis due to asymptomatic thyroid cancer cannot be excluded.
The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for thyroid carcinoma (ver. 2) recommend neck US as a primary diagnostic test in patients with suspected thyroid nodules (19). US-guided FNA or US follow-up should be performed based on the comprehensive result of US finding of the initial scans, together with clinical findings, thyroid-stimulating hormone levels, and thyroglobulin levels.
Ultrasound is a very sensitive method for the detection and diagnosis of thyroid nodules. There is no risk of radiation exposure, and it is also possible to evaluate changes in the thyroid parenchyma and to examine lymph node status. US can also be used to determine the need for US-guided FNA, and improves the accuracy of diagnosis (2324).
However, thyroid nodules are very common and often asymptomatic. No US features that are specific to malignant thyroid nodules have been identified (7). Furthermore, several US findings are evident in both benign and malignant thyroid nodules (202426). This may lead to unnecessary FNA of benign nodules, which may lead to increased medical expenditures and unnecessary complications. However, the cost-effective aspect of the increase in medical expenditures may vary depending on whether or not medical insurance is applied. Finally, the US diagnosis of nodules can cause unnecessary worry and anxiety in patient.
Neck US was chosen as the primary imaging method for patients with suspected or diagnosed thyroid nodule in five medical guidelines. As a result of the evaluation of domestic acceptability and applicability of these five guidelines, it was concluded that the applicability of neck US to the detection and diagnosis of thyroid nodules was reasonable.
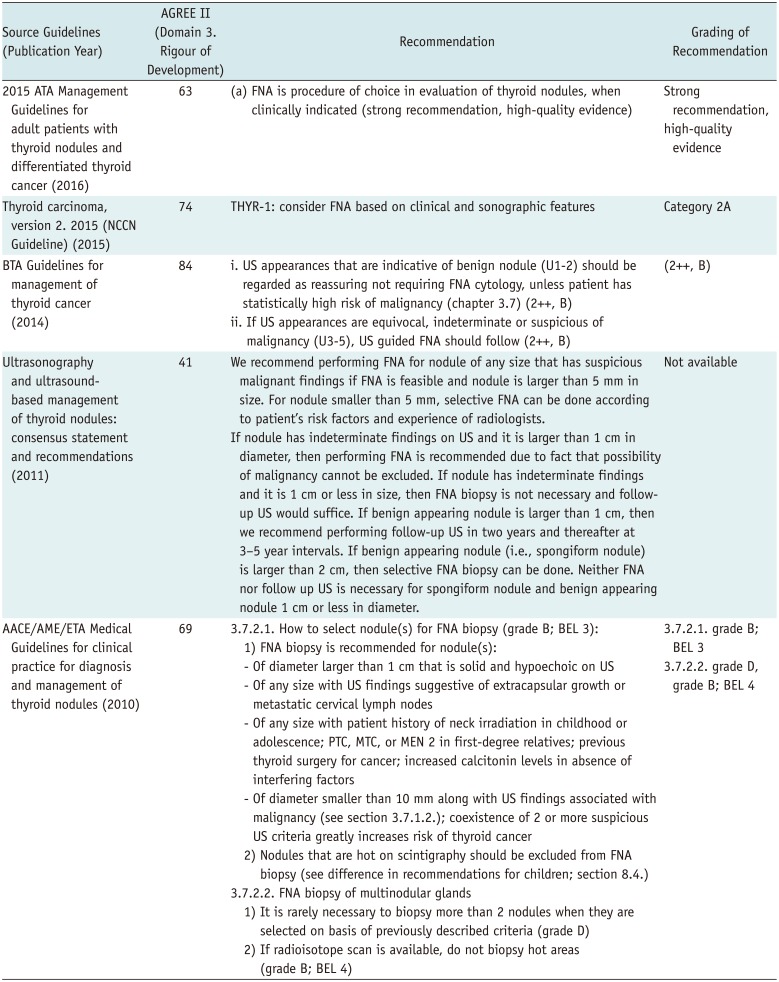
The five final guidelines were selected for appropriate testing methods for histologic diagnosis of thyroid nodules (1316171819). All five guidelines recommend FNA for histologic examination of thyroid nodules. Moreover, according to several comparisons between palpation-guided FNA and US-guided FNA (23272829), the latter is better in terms of non-diagnostic results, specimen errors, and false-negative results.
The BTA Guidelines for the management of thyroid cancer published in 2014 state that FNA is a valuable, cost-effective preoperative test and that US-guided FNA improves the accuracy of diagnosis and reduces the probability of obtaining an inappropriate sample (18). In a prospective study of 215 patients, the non-diagnostic result rate (21.4%) of the US-guided FNA was significantly lower than that of palpation-guided FNA (32.4%), and the false negative result of US-guided FNA (5.6%) was also significantly lower than that of palpation-guided FNA (15.8%) (23).
The ATA Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer strongly recommend FNA as the most accurate and cost-effective test for evaluating thyroid nodules (17). In a retrospective study, US-guided FNA had a significantly low non-diagnostic rate (3.5%) and false-negative rate (1%), compared to palpation-guided FNA (8.7% and 2.3%, respectively) (30). Particularly in cases of cystic, untouchable, or deep-located nodules, US-guided FNA is recommended because of the high probability of non-diagnostic result and sample errors. However, if the nodules are palpable and have a solid component, both US-guided FNA and palpation-guided FNA can be used. The diagnostic sensitivity, specificity, and accuracy of US-guided FNA were 97.1%, 100%, and 75.9%, respectively (2930).
The ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations (13) recommend performing US-guided FNA for histological diagnosis but do not cite any specific evidence.
The AACE/AME/ETA Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules published in 2010 recommend that the diagnosis of thyroid nodules should be based on the results of US and FNA, and that FNA should be done under US guidance to increase reliability and reduce the non-diagnostic result rate (16). US-guided FNA is strongly recommended when nodules are not palpable or are multinodular, when patients are obese, and/or when cervical muscles are highly developed (272830313233). In one prospective study of 386 patients, US-guided FNA showed significantly lower non-diagnostic rate (12.5%) than that of palpation-guided FNA (27.2%) (27).
The NCCN Clinical Practice Guidelines for thyroid carcinoma (ver. 2) recommend that FNA should be performed for histologic diagnosis if the US findings meet the indication (19), particularly when unsuitable specimens or non-diagnostic results are obtained from previous FNAs conducted on solid nodules. However, they cite no specific evidence.
Ultrasound-guided FNA of thyroid nodules is a relatively easy and safe procedure, and is more accurate and less complicated than palpation-guided FNA (23272829). Therefore, it can be performed by any doctor who specializes in thyroid.
However, the rate of non-diagnostic results depends on various factors such as technical proficiency of the practitioner, processing errors, and internal factors of the nodule itself. Therefore, efforts should be made to minimize non-diagnostic results (13). Recent reports have indicated that US-guided CNB may be an alternative to FNA in the case of nodules with non-diagnostic results in previous US-guided FNA (3435). The reported complication rate of US-guided FNA were 0–8.6%, and most complications were hematoma around the thyroid gland, edema of the thyroid gland, and temporary changes in the voice. Severe complications requiring hospitalization were rare. Practitioners should be familiar with the appropriate preparation, prevention, and treatment methods to avoid complications in patients with a bleeding tendency. Unnecessary US-guided FNA for benign nodules can increase patient anxiety, and lead to unnecessary medical expenditures.
These are the first evidence-based clinical imaging guidelines for thyroid imaging in Korea, and was developed using an adaptation process. The guidelines recommend that neck US as the first-line imaging modality for diagnosing thyroid nodules detected by imaging modalities other than US or in patients with suspected thyroid nodules, and US-guided FNA for primary histologic examination of thyroid nodules. We expect that these recommendations will be helpful in the clinical practice in the management of thyroid nodules.
Acknowledgments
The author gratefully acknowledged the KTA for reviewing the final recommendation document.
References
1. Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968; 69:537–540. PMID: 5673172.
2. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf). 1977; 7:481–493. PMID: 598014.

4. Mortensen JD, Woolner LB, Bennett WA. Gross and microscopic findings in clinically normal thyroid glands. J Clin Endocrinol Metab. 1955; 15:1270–1280. PMID: 13263417.

5. Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009; 39:699–706. PMID: 19601965.
6. Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997; 126:226–231. PMID: 9027275.

7. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002; 87:1941–1946. PMID: 11994321.

8. Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf). 2004; 60:21–28. PMID: 14678283.

9. Korean Statistical Information Service. Web site. 2015. Accessed August 25, 2017. http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parmTabId=M_01_01#SubCont.
10. Na DG, Baek JH, Jung SL, Kim JH, Sung JY, Kim KS, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017; 18:217–237. PMID: 28096731.

11. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–395. PMID: 27134526.

12. Choi SJ, Jeong WK, Jo AJ, Choi JA, Kim MJ, Lee M, et al. Methodology for developing evidence-based clinical imaging guidelines: joint recommendations by Korean Society of Radiology and National Evidence-Based Healthcare Collaborating Agency. Korean J Radiol. 2017; 18:208–216. PMID: 28096730.

13. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Korean Society of Thyroid Radiology (KSThR). Korean Society of Radiology. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011; 12:1–14. PMID: 21228935.

14. Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH. Korean Society of Thyroid Radiology (KSThR). Korean Society of Radiology. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the korean society of thyroid radiology. Korean J Radiol. 2015; 16:391–401. PMID: 25741201.

15. Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. Korean Society of Thyroid Radiology (KSThR). Korean Society of Radiology. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012; 13:117–125. PMID: 22438678.

16. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2010; 16(Suppl 1):1–43.
17. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.

18. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. British Thyroid Association. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014; 81(Suppl 1):1–122.

19. National Comprehensive Cancer Network. Web site. 2015. Accessed August 14, 2015. https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.
20. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Society of Radiologists in Ultrasound. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005; 237:794–800. PMID: 16304103.

21. Smith-Bindman R, Lebda P, Feldstein VA, Sellami D, Goldstein RB, Brasic N, et al. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: results of a population-based study. JAMA Intern Med. 2013; 173:1788–1796. PMID: 23978950.
22. Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014; 99:1253–1263. PMID: 24276450.

23. Cesur M, Corapcioglu D, Bulut S, Gursoy A, Yilmaz AE, Erdogan N, et al. Comparison of palpation-guided fine-needle aspiration biopsy to ultrasound-guided fine-needle aspiration biopsy in the evaluation of thyroid nodules. Thyroid. 2006; 16:555–561. PMID: 16839257.

24. Hambly NM, Gonen M, Gerst SR, Li D, Jia X, Mironov S, et al. Implementation of evidence-based guidelines for thyroid nodule biopsy: a model for establishment of practice standards. AJR Am J Roentgenol. 2011; 196:655–660. PMID: 21343510.

25. Solbiati L, Osti V, Cova L, Tonolini M. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur Radiol. 2001; 11:2411–2424. PMID: 11734934.

26. Lee YH, Kim DW, In HS, Park JS, Kim SH, Eom JW, et al. Differentiation between benign and malignant solid thyroid nodules using an US classification system. Korean J Radiol. 2011; 12:559–567. PMID: 21927557.

27. Can AS, Peker K. Comparison of palpation-versus ultrasound-guided fine-needle aspiration biopsies in the evaluation of thyroid nodules. BMC Res Notes. 2008; 1:12. PMID: 18710537.

28. Deandrea M, Mormile A, Veglio M, Motta M, Pellerito R, Gallone G, et al. Fine-needle aspiration biopsy of the thyroid: comparison between thyroid palpation and ultrasonography. Endocr Pract. 2002; 8:282–286. PMID: 12185993.

29. Carmeci C, Jeffrey RB, McDougall IR, Nowels KW, Weigel RJ. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998; 8:283–289. PMID: 9588492.

30. Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998; 8:15–21. PMID: 9492148.

31. Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007; 111:306–315. PMID: 17680588.

32. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007; 36:707–735. viPMID: 17673125.

33. Wu HH, Jones JN, Osman J. Fine-needle aspiration cytology of the thyroid: ten years experience in a community teaching hospital. Diagn Cytopathol. 2006; 34:93–96. PMID: 16514671.

34. Baek JH. Current status of core needle biopsy of the thyroid. Ultrasonography. 2017; 36:83–85. PMID: 28301922.

35. Suh CH, Baek JH, Kim KW, Sung TY, Kim TY, Song DE, et al. The role of core-needle biopsy for thyroid nodules with initially nondiagnostic fine-needle aspiration results: a systematic review and meta-analysis. Endocr Pract. 2016; 22:679–688. PMID: 27176143.

Table 1
Recommendation Matrix of Existing Guidelines (Key Question 1)
AACE = American Association of Clinical Endocrinologists, AGREE = Appraisal of Guidelines for Research & Evaluation, AME = Associazione Medici Endocrinologi, ATA = American Thyroid Association, BEL = best evidence level, BTA = British Thyroid Association, ETA = European Thyroid Association, FNA = fine-needle aspiration, MNG = multinodular goiter, NCCN = National Comprehensive Cancer Network, US = ultrasound
Table 2
Recommendation Matrix of Existing Guidelines (Key Question 2)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download