Abstract
Objective
To compare the safety and efficacy between a covered metallic ureteral stent (CMS) and a double-J ureteral stent (DJS) for the treatment of a malignant ureteral obstruction (MUO).
Materials and Methods
Nineteen patients (seven men and 12 women; mean age, 53.4 years) were randomly assigned to the CMS (n = 10) or DJS (n = 9) group. The following were compared between the two groups: technical success, i.e., successful stent placement into desired locations; stent malfunction; stent patency, i.e., no obstruction and no additional intervention; complications; and patient survival.
Results
The technical success rate was 100% in all 10 and 12 ureteral units in the CMS and DJS groups, respectively. During the mean follow-up period of 253.9 days (range, 63–655 days), stent malfunction was observed in 40.0% (4/10) and 66.7% (8/12) in the CMS and DJS groups, respectively. In the per-ureteral analysis, the median patency time was 239.0 days and 80.0 days in the CMS and DJS groups, respectively. The CMS group yielded higher patency rates compared with the DJS group at three months (90% vs. 35%) and at six months (57% vs. 21%). The overall patency rates were significantly higher in the CMS group (p = 0.041). Complications included the migration of two metallic stents in one patient in the CMS group, which were removed in a retrograde manner. The two patient groups did not differ significantly regarding their overall survival rates (p = 0.286).
Malignant ureteral obstruction (MUO) can result from a variety of malignancies and lead to urosepsis or renal impairment (1). MUO is generally associated with a short life expectancy and significantly diminishes the quality of life (QoL) (2). Accordingly, efforts are made to maintain renal function as well as QoL. To date, percutaneous nephrostomy (PCN) and/or a double-J ureteral stent (DJS) have been widely used to relieve MUO. However, the externally placed PCN tubes cause significant patient discomfort and deteriorate the QoL despite the rapid relief from MUO (34). While DJS is more comfortable for patients, it has drawbacks including the necessity of repetitive exchange, bladder irritation, and a high incidence of stent malfunction (567).
Recently, various types of metallic ureteral stents have been suggested to effectively manage MUO and to overcome the drawbacks of PCN and DJS. (89101112). Of them, covered metallic ureteral stents (CMSs) have many advantages compared with bare metallic ureteral stents, such as a lower rate of urothelial hyperplasia and higher patency rate (13141516). Despite the promising results of CMSs, most of the studies are retrospectively based on personal, clinical experience. Notably, comparative studies of both the DJS and CMS are rare (1718). Therefore, the purpose of this study was to prospectively compare the safety and efficacy between the CMS and DJS for the treatment of MUO.
This study was approved by the Institutional Review Board of Asan Medical Center. Patients were informed of the risks and benefits of participation in the study and each provided written, informed consent before being enrolled. This trial is registered at ClinicalTrials.gov, number NCT01823575. Between May 2013 and March 2016, 19 patients (seven men and 12 women; mean age, 53.4 years) with MUO were randomly assigned using simple randomization to the CMS (n = 10, 10 ureteral units) or DJS (n = 9, 12 ureteral units) group. The inclusion criteria included patients 20–80 years of age and a MUO caused by an abdominal or pelvic malignancy with overt hydronephrosis that was documented on ultrasonography (US) or computed tomography (CT) scans. The exclusion criteria were as follows: 1) expected patient life expectancy of less than three months; 2) history of unilateral nephrectomy or bladder reconstruction; 3) past kidney transplantation; 4) history of severe allergies to contrast media; 5) state of dialysis; and 6) a performance status of 3 or 4 according to the Eastern Cooperative Oncology Group performance status scale. The baseline patients' characteristics and disease etiologies are listed in Table 1.
The study endpoints included the primary patency rates at 3, 6, and 12 months. The confirmation of patency of the CMS or DJS was made according to renal biochemistry or imaging studies such as US or CT scan. The primary patency rate was defined as the time period from the initial stent insertion to either recurrent hydronephrosis as confirmed by follow-up imaging studies or secondary intervention to relieve the ureteral reocclusion. If stent dysfunction was not evident during a patient's lifetime, the stent patency was considered as equal to the time of the patient's survival. The following were also compared between the two patient groups: technical success, stent malfunction, complications, and survival rates. Technical success was defined as a successful stent placement into a desired location. Complications were classified as minor or major according to the Society of Interventional Radiology guidelines (19). Patient survival was defined as the period of time between the initial stent placement and the patient's death.
After PCN, an antegrade nephrogram was obtained for the evaluation of the level and length of the obstruction. A 9 Fr introducer sheath (Pinnacle TIF Tip; Terumo, Tokyo, Japan) was inserted over the 0.035-inch, hydrophilic stiff guidewire (Radifocus; Terumo) after removing the PCN tube. A 5 Fr catheter (Kumpe; Cook, Bloomington, IN, USA) was inserted over the guidewire for traversal of the obstructed ureter into the urinary bladder. Subsequently, after removing the catheter, the obstructed ureteral segment was routinely dilated by using a 6-mm balloon catheter (Ascend; Cook). Finally, an 8 Fr, DJS (Flexima; Boston Scientific, Natick, MA, USA) was inserted with the proximal end in the renal pelvis and the distal end within the urinary bladder.
The placement of a CMS (Urexel stent; S&G Biotech, Seongnam, Korea) was conducted in the same manner as the placement of a DJS until the point of pre-stenting ballooning. The CMS was internally fully coated with a thin silicone membrane and tapered distally (Fig. 1). The stent loaded in an 8 Fr stent introducer was 7 mm in diameter and ranged in length from 10 cm to 16 cm. The stent was deployed by pulling back the introducer sheath. If more than two stents were placed, due to multiple or long obstructed segments, the stents were placed from the renal pelvis across the ureterovesical junction (UVJ) and overlapped by 3–4 cm in order to prevent stent migration or separation. Post-stent balloon dilatation was performed using a 6-mm balloon catheter if the stent did not achieve its expected maximum diameter.
After placing the stents, another PCN tube was inserted in order to determine the patency of the stents and was subsequently clamped when the drained urine was clear or showed mild hematuria. Finally, the PCN tube was removed two days after the procedure if the nephrogram confirmed good patency and satisfactory positions of the stents or if patients did not have any symptoms or signs such as flank pain, fever or urine leakage via the PCN tract.
All patients were clinically evaluated using renal biochemistry, e.g. blood urea nitrogen (BUN) or serum creatinine (Cr), plain abdominal radiography, and kidney US at 1, 3, 6, 9, and 12 months post-procedure. Patients with unexpected symptoms such as urinary frequency, flank pain, dysuria, hematuria or fever were evaluated on an urgent basis. In order to evaluate the stent patency and patient survival, telephone interviews were performed monthly after the initial 12 months of follow-up and until the patient's death. Complications were diagnosed using clinical and laboratory examinations as well as imaging.
Continuous variables are presented as mean ± standard deviation and were compared by Student t tests. The categorical variables were compared by a chi-square test or Fisher's exact test. The primary patency rates and survival rates were assessed according to the Kaplan-Meier method and compared by the log-rank test. A p value of less than 0.05 was considered to indicate statistical significance. The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS 21.0 version) software (IBM Corp, Armonk, NY, USA).
The technical success was 100% for each group, which were specifically 10 ureteral units in 10 CMS group patients and 12 ureteral units in nine DJS group patients. All of the CMS group patients received trans UVJ stenting, of which there were two stents with overlap stenting placed in nine patients and a single stent in one patient (Fig. 2). In the DJS group, three patients had bilateral ureteral involvement; thus, they were treated by bilateral, double-J ureteral stenting. None of the patients experienced immediate stent failure within a few days after the procedure. In all of the patients of both groups, their renal biochemistries, including the levels of BUN/Cr, were stabilized after the procedures, and the PCN tubes were removed. There were no immediate procedure-related complications.
All patients were followed up until their death. During the mean follow-up period of 253.9 days (range, 63–655 days), stent malfunction occurred in 40.0% (4/10) and 66.7% (8/12) of the CMS and DJS patient groups, respectively. The migration of metallic stents in one patient in the CMS group occurred 34 days following stent placement, and they were removed in a retrograde manner followed by a DJS insertion. In the per-ureteral analysis, the median patency time for the CMS and DJS groups were 239.0 days and 80.0 days, respectively. The cumulative patency rates at 3, 6, 9, and 12 months were 90%, 57%, 38%, and 38% in the CMS group and 35%, 21%, 21%, and 21% in the DJS group, respectively. The overall patency rates between the two groups were statistically significant (p = 0.041) (Fig. 3). The median survival for the CMS and DJS groups were 203.0 days and 194.0 days, respectively. The two groups did not differ significantly in the overall survival rates (p = 0.286) (Fig. 4).
A prospective, randomized, comparative study regarding the efficacy and safety of DJSs and CMSs was conducted. In this study, the silicone-covered, metallic, ureteral stents were used because the silicone membrane has a high degree of tissue compatibility, flexibility, and resistance to encrustation (20). Unlike the uncovered, metallic stents, CMSs tend to have longer patency by preventing tissue or tumor ingrowth through the struts of the stents. Previous experimental studies have already demonstrated the positive effect of the covered stent for preventing tissue ingrowth (1321). In addition, several previous studies established the superior results of a polytetrafluoroethylene-covered, self-expandable metallic stent (UVENTA; Taewoong Medical, Seoul, Korea) in terms of the primary patency (10141517). This study also demonstrated that silicone-covered, metallic, ureteral stents yielded higher primary patency rates at 90 days and 180 days than the DJSs (90% vs. 35% and 57% vs. 21%). The stents used in this study had a large lumen which allowed enough urine flow to effectively remove the possible core of stones from the lumen. Conversely, since DJSs have narrow lumens with small holes, the effective cleaning of the lumen is, therefore, not possible. Furthermore, it can be easily occluded with even minute amounts of stone in the narrow lumen (17).
Specifically, in this study, two metallic, ureteral stents were placed across the UVJ with a 3–4-cm overlap from the renal pelvis to the urinary bladder in order to prevent stent migration and edge-stenosis that is caused by urothelial hyperplasia at the margin of the stent or periureteral spread of the tumor as well as to cover multiple or long-segmental strictures. This stenting approach can also be one of the primary reasons for the superior primary patency rates of the CMS group. Accordingly, the longer patency of the CMS group will allow infrequent secondary interventions such as PCN or another stent placement.
However, migration is a major issue limiting the widespread clinical use of CMSs because the ureter has a peristaltic movement that allows for the maintenance of the urinary flow and adherence to the ureter lumen can be lost with the covered membrane (1122). Interestingly, in this study, stent migration occurred in one patient at 34 days after stent placement due to insufficient overlap between the two stents and not considering longitudinal stent-shortening. However, it was assumed that the CMS applied in this study can effectively prevent migration because of its flared structure that is encircled with an outer bare mesh at the proximal 1-cm end.
Comparing various metallic stents is complicated when using published data because the nature of the underlying malignancy and life expectancy of patients can affect the patency rate. Also, the definitions of patency rates and specific procedures vary among studies. In fact, all of the patients enrolled in our study exhibited multiple or long segmental, severe strictures or obstructions caused by aggressive and progressive malignancies.
Of note, Kim et al. (23) expressed serious concern regarding the disappointing long-term outcomes of metallic ureteral stents (UVENTA) in terms of major complications (28%, median 27.1 months). Direct ureteral injury with or without fistula formation to adjacent organs, including ureteroarterial fistulas, was the most common major complications. Identified risk factors for major complications were a history of cervical cancer and radiation therapy. However, in this study, there were no such life-threatening major complications; although, two patients with a history of gynecologic cancer and previous pelvic radiation therapy were included.
Although CMS seems to be an alternative to DJS for MUO, no definitive indication currently exists regarding how and which metal stent should be used in a specific MUO. According to the current study, patients with advanced gastric cancer that are experiencing rapid progression with periureteral spread and/or having one year or less of life expectancy are good candidates for the metallic ureteral stenting in the palliative setting.
This study has a strong point in that it is the first randomized study comparing the efficacy and safety between CMSs and DJSs. However, this study has several limitations. First, the enrolled number of patients was small. Second, endoscopic examination with a pathology biopsy for the occluded stents was not performed in this study. Therefore, the exact cause of the occlusion could not be evaluated pathologically. Finally, the economic impact was not evaluated and the cost-effectiveness of metallic ureteral stenting if the patient has a reasonable life expectancy was not considered.
In conclusion, CMS may be effective for MUO. A multi-center study with a larger patient cohort is warranted to further assess the outcomes of CMSs for MUO.
Figures and Tables
Fig. 1
Structure of silicone-covered, self-expandable, metallic stent (Urexel, S&G Biotech).
A. Both ends are fully covered with silicone membrane, and distally tapered shape is shown with 1-mm difference in diameter between proximal and distal ends. B. Comparison of Urexel metallic ureteral stent and DJS. DJS = double-J ureteral stent

Fig. 2
55-year-old, male patient with advanced gastric cancer.
A. Enhanced axial CT image shows hydronephrosis of left kidney (arrow) that was caused by periureteric metastasis. B. Antegrade pyelogram via percutaneous nephrostomy tube shows diffuse, long-segmental ureteric strictures (arrows) from proximal- to mid-ureter. C. Antegrade pyelogram immediately following metallic ureteral stents placement shows good flow of contrast medium through metallic ureteral stents into urinary bladder. D. Follow-up enhanced CT image obtained one month after metallic ureteral stents placement shows complete resolution of hydronephrosis of left kidney (arrow). CT = computed tomography
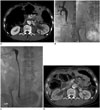
Fig. 3
Kaplan-Meier curve of primary patency rates of both stent groups.
Overall patency rates were significantly higher in CMS group compared with DJS group (log-rank test, p = 0.041). CMS = covered metallic ureteral stent

Fig. 4
Kaplan-Meier curve of survival rates of both stent groups.
Two groups did not differ significantly regarding overall survival rates (log-rank test, p = 0.286).

Table 1
Baseline Patient Characteristics in Two Groups

References
1. Better OS, Arieff AI, Massry SG, Kleeman CR, Maxwell MH. Studies on renal function after relief of complete unilateral ureteral obstruction of three months' duration in man. Am J Med. 1973; 54:234–240.

2. Russo P. Urologic emergencies in the cancer patient. Semin Oncol. 2000; 27:284–298.
3. Farrell TA, Hicks ME. A review of radiologically guided percutaneous nephrostomies in 303 patients. J Vasc Interv Radiol. 1997; 8:769–774.

4. Radecka E, Magnusson A. Complications associated with percutaneous nephrostomies. A retrospective study. Acta Radiol. 2004; 45:184–188.

5. Saltzman B. Ureteral stents. Indications, variations, and complications. Urol Clin North Am. 1988; 15:481–491.
6. Chung SY, Stein RJ, Landsittel D, Davies BJ, Cuellar DC, Hrebinko RL, et al. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. J Urol. 2004; 172:592–595.

7. Docimo SG, Dewolf WC. High failure rate of indwelling ureteral stents in patients with extrinsic obstruction: experience at 2 institutions. J Urol. 1989; 142(2 Pt 1):277–279.

8. Lang EK, Winer AG, Abbey-Mensah G, Anne R, Allaei A, Friedman F, et al. Long-term results of metallic stents for malignant ureteral obstruction in advanced cervical carcinoma. J Endourol. 2013; 27:646–651.

9. Wang HJ, Lee TY, Luo HL, Chen CH, Shen YC, Chuang YC, et al. Application of resonance metallic stents for ureteral obstruction. BJU Int. 2011; 108:428–432.

10. Kim JH, Song K, Jo MK, Park JW. Palliative care of malignant ureteral obstruction with polytetrafluoroethylene membrane-covered self-expandable metallic stents: initial experience. Korean J Urol. 2012; 53:625–631.

11. Trueba Arguiñarena FJ, Fernández del Busto E. Self-expanding polytetrafluoroethylene covered nitinol stents for the treatment of ureteral stenosis: preliminary report. J Urol. 2004; 172:620–623.
12. Pollak JS, Rosenblatt MM, Egglin TK, Dickey KW, Glickman M. Treatment of ureteral obstructions with the Wallstent endoprosthesis: preliminary results. J Vasc Interv Radiol. 1995; 6:417–425.

13. Chung HH, Lee SH, Cho SB, Park HS, Kim YS, Kang BC, et al. Comparison of a new polytetrafluoroethylene-covered metallic stent to a noncovered stent in canine ureters. Cardiovasc Intervent Radiol. 2008; 31:619–628.

14. Chung KJ, Park BH, Park B, Lee JH, Kim WJ, Baek M, et al. Efficacy and safety of a novel, double-layered, coated, self-expandable metallic mesh stent (Uventa™) in malignant ureteral obstructions. J Endourol. 2013; 27:930–935.

15. Kim KS, Choi S, Choi YS, Bae WJ, Hong SH, Lee JY, et al. Comparison of efficacy and safety between a segmental thermo-expandable metal alloy spiral stent (Memokath 051) and a self-expandable covered metallic stent (UVENTA) in the management of ureteral obstructions. J Laparoendosc Adv Surg Tech A. 2014; 24:550–555.

16. Kim KH, Cho KS, Ham WS, Hong SJ, Han KS. Early application of permanent metallic mesh stent in substitution for temporary polymeric ureteral stent reduces unnecessary ureteral procedures in patients with malignant ureteral obstruction. Urology. 2015; 86:459–464.

17. Chung HH, Kim MD, Won JY, Won JH, Cho SB, Seo TS, et al. Multicenter experience of the newly designed covered metallic ureteral stent for malignant ureteral occlusion: comparison with double J stent insertion. Cardiovasc Intervent Radiol. 2014; 37:463–470.

18. Chow PM, Chiang IN, Chen CY, Huang KH, Hsu JS, Wang SM, et al. Malignant Ureteral Obstruction: Functional Duration of Metallic versus Polymeric Ureteral Stents. PLoS One. 2015; 10:e0135566.

19. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S199–S202.

20. Denstedt JD, Wollin TA, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998; 12:493–500.

21. Liatsikos EN, Karnabatidis D, Katsanos K, Kallidonis P, Katsakiori P, Kagadis GC, et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. J Urol. 2009; 182:2613–2617.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download