1. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010; 110:1368–1373. PMID:
20237047.

2. Delotte J, Novellas S, Koh C, Bongain A, Chevallier P. Obstetrical prognosis and pregnancy outcome following pelvic arterial embolisation for post-partum hemorrhage. Eur J Obstet Gynecol Reprod Biol. 2009; 145:129–132. PMID:
19398259.

3. Deneux-Tharaux C, Dupont C, Colin C, Rabilloud M, Touzet S, Lansac J, et al. Multifaceted intervention to decrease the rate of severe postpartum haemorrhage: the PITHAGORE6 cluster-randomised controlled trial. BJOG. 2010; 117:1278–1287. PMID:
20573150.

4. Sentilhes L, Vayssière C, Deneux-Tharaux C, Aya AG, Bayoumeu F, Bonnet MP, et al. Postpartum hemorrhage: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF): in collaboration with the French Society of Anesthesiology and Intensive Care (SFAR). Eur J Obstet Gynecol Reprod Biol. 2016; 198:12–21. PMID:
26773243.
5. Sheldon WR, Blum J, Vogel JP, Souza JP, Gülmezoglu AM, Winikoff B. WHO Multicountry Survey on Maternal and Newborn Health Research Network. Postpartum haemorrhage management, risks, and maternal outcomes: findings from the World Health Organization multicountry survey on maternal and newborn health. BJOG. 2014; 121(Suppl 1):5–13. PMID:
24641530.

6. Gonsalves M, Belli A. The role of interventional radiology in obstetric hemorrhage. Cardiovasc Intervent Radiol. 2010; 33:887–895. PMID:
20464555.

7. Soyer P, Dohan A, Dautry R, Guerrache Y, Ricbourg A, Gayat E, et al. Transcatheter arterial embolization for postpartum hemorrhage: indications, technique, results, and complications. Cardiovasc Intervent Radiol. 2015; 38:1068–1081. PMID:
25677130.

8. Palacios Jaraquemada JM, García Mónaco R, Barbosa NE, Ferle L, Iriarte H, Conesa HA. Lower uterine blood supply: extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet Gynecol Scand. 2007; 86:228–234. PMID:
17364288.

9. Chang S, Lee MS, Kim MD, Yoon CJ, Jung DC, Lee M, et al. Inferior mesenteric artery collaterals to the uterus during uterine artery embolization: prevalence, risk factors, and clinical outcomes. J Vasc Interv Radiol. 2013; 24:1353–1360. PMID:
23891048.

10. Ojala K, Perälä J, Kariniemi J, Ranta P, Raudaskoski T, Tekay A. Arterial embolization and prophylactic catheterization for the treatment for severe obstetric hemorrhage*. Acta Obstet Gynecol Scand. 2005; 84:1075–1080. PMID:
16232175.

11. Lee HJ, Jeon GS, Kim MD, Kim SH, Lee JT, Choi MJ. Usefulness of pelvic artery embolization in cesarean section compared with vaginal delivery in 176 patients. J Vasc Interv Radiol. 2013; 24:103–109. PMID:
23273701.

12. Soyer P, Boudiaf M, Jacob D, Hamzi L, Pelage JP, Le Dref O, et al. Bilateral persistent sciatic artery: a potential risk in pelvic arterial embolization for primary postpartum hemorrhage. Acta Obstet Gynecol Scand. 2005; 84:604–605. PMID:
15901276.

13. van Hooft IM, Zeebregts CJ, van Sterkenburg SM, de Vries WR, Reijnen MM. The persistent sciatic artery. Eur J Vasc Endovasc Surg. 2009; 37:585–591. PMID:
19231248.

14. Gipson MG, Smith MT. Endovascular therapies for primary postpartum hemorrhage: techniques and outcomes. Semin Intervent Radiol. 2013; 30:333–339. PMID:
24436559.

15. Park KJ, Shin JH, Yoon HK, Gwon DI, Ko GY, Sung KB. Postpartum hemorrhage from extravasation or pseudoaneurysm: efficacy of transcatheter arterial embolization using N-butyl cyanoacrylate and comparison with gelatin sponge particle. J Vasc Interv Radiol. 2015; 26:154–161. PMID:
25454736.

16. Worthington-Kirsch RL. Uterine artery embolization: state of the art. Semin Intervent Radiol. 2004; 21:37–42. PMID:
21331107.

17. Leleup G, Fohlen A, Dohan A, Bryan-Rest L, Le Pennec V, Limot O, et al. Value of round ligament artery embolization in the management of postpartum hemorrhage. J Vasc Interv Radiol. 2017; 28:696–701. PMID:
28292635.

18. Wi JY, Kim HC, Chung JW, Jun JK, Jae HJ, Park JH. Importance of angiographic visualization of round ligament arteries in women evaluated for intractable vaginal bleeding after uterine artery embolization. J Vasc Interv Radiol. 2009; 20:1031–1035. PMID:
19560937.

19. Lee HY, Shin JH, Kim J, Yoon HK, Ko GY, Won HS, et al. Primary postpartum hemorrhage: outcome of pelvic arterial embolization in 251 patients at a single institution. Radiology. 2012; 264:903–909. PMID:
22829685.

20. Kirby JM, Kachura JR, Rajan DK, Sniderman KW, Simons ME, Windrim RC, et al. Arterial embolization for primary postpartum hemorrhage. J Vasc Interv Radiol. 2009; 20:1036–1045. PMID:
19647182.

21. Chauleur C, Fanget C, Tourne G, Levy R, Larchez C, Seffert P. Serious primary post-partum hemorrhage, arterial embolization and future fertility: a retrospective study of 46 cases. Hum Reprod. 2008; 23:1553–1559. PMID:
18460450.

22. Ganguli S, Stecker MS, Pyne D, Baum RA, Fan CM. Uterine artery embolization in the treatment of postpartum uterine hemorrhage. J Vasc Interv Radiol. 2011; 22:169–176. PMID:
21183360.

23. Olowokere AE, Adekeye OA, Ogunfowokan A, Olagunju OE, Irinoye OO. The prevalence, management and outcome of primary postpartum haemorrhage in selected health care facilities in Nigeria. Int J Nurs Midwifery. 2013; 5:28–34.

24. Gizzo S, Saccardi C, Patrelli TS, Di Gangi S, Breda E, Fagherazzi S, et al. Fertility rate and subsequent pregnancy outcomes after conservative surgical techniques in postpartum hemorrhage: 15 years of literature. Fertil Steril. 2013; 99:2097–2107. PMID:
23498891.

25. Healey S, Buzaglo K, Seti L, Valenti D, Tulandi T. Ovarian function after uterine artery embolization and hysterectomy. J Am Assoc Gynecol Laparosc. 2004; 11:348–352. PMID:
15559347.

26. Machado LS. Emergency peripartum hysterectomy: incidence, indications, risk factors and outcome. N Am J Med Sci. 2011; 3:358–361. PMID:
22171242.

27. Ko HK, Shin JH, Ko GY, Gwon DI, Kim JH, Han K, et al. Efficacy of prophylactic uterine artery embolization before obstetrical procedures with high risk for massive bleeding. Korean J Radiol. 2017; 18:355–360. PMID:
28246515.

28. Cali G, Forlani F, Giambanco L, Amico ML, Vallone M, Puccio G, et al. Prophylactic use of intravascular balloon catheters in women with placenta accreta, increta and percreta. Eur J Obstet Gynecol Reprod Biol. 2014; 179:36–41. PMID:
24965977.

29. Yi KW, Oh MJ, Seo TS, So KA, Paek YC, Kim HJ. Prophylactic hypogastric artery ballooning in a patient with complete placenta previa and increta. J Korean Med Sci. 2010; 25:651–655. PMID:
20358016.

30. Fuchs KM, Miller RS, Berkowitz RL. Optimizing outcomes through protocols, multidisciplinary drills, and simulation. Semin Perinatol. 2009; 33:104–108. PMID:
19324239.

31. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006; 108:1039–1047. PMID:
17012482.
32. Matsubara S, Sato T, Nakata M. Vaginal artery embolization with a permanent embolic agent for intractable postpartum hemorrhage. J Obstet Gynaecol Res. 2011; 37:377–378. PMID:
21418425.

33. Kim GM, Yoon CJ, Seong NJ, Kang SG, Kim YJ. Postpartum haemorrhage from ruptured pseudoaneurysm: efficacy of transcatheter arterial embolisation using N-butyl-2-cyanoacrylate. Eur Radiol. 2013; 23:2344–2349. PMID:
23559143.

34. Obata S, Kasai M, Kasai J, Seki K, Sekikawa Z, Torimoto I, et al. Emergent uterine arterial embolization using N-butyl cyanoacrylate in postpartum hemorrhage with disseminated intravascular coagulation. Biomed Res Int. 2017; 2017:1562432. PMID:
28251148.

35. Cottier JP, Fignon A, Tranquart F, Herbreteau D. Uterine necrosis after arterial embolization for postpartum hemorrhage. Obstet Gynecol. 2002; 100(5 Pt 2):1074–1077. PMID:
12423810.

36. Park HS, Shin JH, Yoon HK, Kim JH, Gwon DI, Ko GY, et al. Transcatheter arterial embolization for secondary postpartum hemorrhage: outcome in 52 patients at a single tertiary referral center. J Vasc Interv Radiol. 2014; 25:1751–1757. PMID:
24985718.

37. Dohan A, Soyer P, Subhani A, Hequet D, Fargeaudou Y, Morel O, et al. Postpartum hemorrhage resulting from pelvic pseudoaneurysm: a retrospective analysis of 588 consecutive cases treated by arterial embolization. Cardiovasc Intervent Radiol. 2013; 36:1247–1255. PMID:
23756881.

38. McGonegle SJ, Dziedzic TS, Thomas J, Hertzberg BS. Pseudoaneurysm of the uterine artery after an uncomplicated spontaneous vaginal delivery. J Ultrasound Med. 2006; 25:1593–1597. PMID:
17121956.

39. Sharma AM, Burbridge BE. Uterine artery pseudoaneurysm in the setting of delayed postpartum hemorrhage: successful treatment with emergency arterial embolization. Case Rep Radiol. 2011; 2011:373482. PMID:
22606544.

40. Kim YJ, Yoon CJ, Seong NJ, Kang SG, An SW, Kim YS, et al. Failed pelvic arterial embolization for postpartum hemorrhage: clinical outcomes and predictive factors. J Vasc Interv Radiol. 2013; 24:703–709. PMID:
23622042.

41. Doumouchtsis SK, Nikolopoulos K, Talaulikar V, Krishna A, Arulkumaran S. Menstrual and fertility outcomes following the surgical management of postpartum haemorrhage: a systematic review. BJOG. 2014; 121:382–388. PMID:
24321038.

42. Sentilhes L, Gromez A, Clavier E, Resch B, Verspyck E, Marpeau L. Fertility and pregnancy following pelvic arterial embolisation for postpartum haemorrhage. BJOG. 2010; 117:84–93. PMID:
19832826.

43. Hardeman S, Decroisette E, Marin B, Vincelot A, Aubard Y, Pouquet M, et al. Fertility after embolization of the uterine arteries to treat obstetrical hemorrhage: a review of 53 cases. Fertil Steril. 2010; 94:2574–2579. PMID:
20381035.

44. Eriksson LG, Mulic-Lutvica A, Jangland L, Nyman R. Massive postpartum hemorrhage treated with transcatheter arterial embolization: technical aspects and long-term effects on fertility and menstrual cycle. Acta Radiol. 2007; 48:635–642. PMID:
17611871.

45. Nikolic B, Spies JB, Campbell L, Walsh SM, Abbara S, Lundsten MJ. Uterine artery embolization: reduced radiation with refined technique. J Vasc Interv Radiol. 2001; 12:39–44. PMID:
11200352.

46. Andrews RT, Brown PH. Uterine arterial embolization: factors influencing patient radiation exposure. Radiology. 2000; 217:713–722. PMID:
11110933.

47. Tse G, Spies JB. Radiation exposure and uterine artery embolization: current risks and risk reduction. Tech Vasc Interv Radiol. 2010; 13:148–153. PMID:
20723828.

48. Salomon LJ, deTayrac R, Castaigne-Meary V, Audibert F, Musset D, Ciorascu R, et al. Fertility and pregnancy outcome following pelvic arterial embolization for severe post-partum haemorrhage. A cohort study. Hum Reprod. 2003; 18:849–852. PMID:
12660283.

49. Fargeaudou Y, Soyer P, Morel O, Sirol M, le Dref O, Boudiaf M, et al. Severe primary postpartum hemorrhage due to genital tract laceration after operative vaginal delivery: successful treatment with transcatheter arterial embolization. Eur Radiol. 2009; 19:2197–2203. PMID:
19415291.

50. Soyer P, Morel O, Fargeaudou Y, Sirol M, Staub F, Boudiaf M, et al. Value of pelvic embolization in the management of severe postpartum hemorrhage due to placenta accreta, increta or percreta. Eur J Radiol. 2011; 80:729–735. PMID:
20708361.

51. Izbizky G, Meller C, Grasso M, Velazco A, Peralta O, Otaño L, et al. Feasibility and safety of prophylactic uterine artery catheterization and embolization in the management of placenta accreta. J Vasc Interv Radiol. 2015; 26:162–169. quiz 170. PMID:
25533451.

52. Shrivastava V, Nageotte M, Major C, Haydon M, Wing D. Case-control comparison of cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol. 2007; 197:402.e1. 402.e5. PMID:
17904978.

53. Yu PC, Ou HY, Tsang LL, Kung FT, Hsu TY, Cheng YF. Prophylactic intraoperative uterine artery embolization to control hemorrhage in abnormal placentation during late gestation. Fertil Steril. 2009; 91:1951–1955. PMID:
18501901.

54. Hequet D, Morel O, Soyer P, Gayat E, Malartic C, Barranger E. Delayed hysteroscopic resection of retained tissues and uterine conservation after conservative treatment for placenta accreta. Aust N Z J Obstet Gynaecol. 2013; 53:580–583. PMID:
24138386.
55. Diop AN, Chabrot P, Bertrand A, Constantin JM, Cassagnes L, Storme B, et al. Placenta accreta: management with uterine artery embolization in 17 cases. J Vasc Interv Radiol. 2010; 21:644–648. PMID:
20227296.

56. Uchiyama D, Koganemaru M, Abe T, Hori D, Hayabuchi N. Arterial catheterization and embolization for management of emergent or anticipated massive obstetrical hemorrhage. Radiat Med. 2008; 26:188–197. PMID:
18509718.

57. Wang Z, Li X, Pan J, Zhang X, Shi H, Yang N, et al. Uterine artery embolization for management of primary postpartum hemorrhage associated with placenta accreta. Chin Med Sci J. 2016; 31:228–232. PMID:
28065219.

58. Kim T, Shin JH, Kim J, Yoon HK, Ko GY, Gwon DI, et al. Management of bleeding uterine arteriovenous malformation with bilateral uterine artery embolization. Yonsei Med J. 2014; 55:367–373. PMID:
24532505.

59. Aziz N, Lenzi TA, Jeffrey RB Jr, Lyell DJ. Postpartum uterine arteriovenous fistula. Obstet Gynecol. 2004; 103(5 Pt 2):1076–1078. PMID:
15121613.

60. Maleux G, Timmerman D, Heye S, Wilms G. Acquired uterine vascular malformations: radiological and clinical outcome after transcatheter embolotherapy. Eur Radiol. 2006; 16:299–306. PMID:
15977019.

61. Pelage JP, Le Dref O, Mateo J, Soyer P, Jacob D, Kardache M, et al. Life-threatening primary postpartum hemorrhage: treatment with emergency selective arterial embolization. Radiology. 1998; 208:359–362. PMID:
9680559.

62. Touboul C, Badiou W, Saada J, Pelage JP, Payen D, Vicaut E, et al. Efficacy of selective arterial embolisation for the treatment of life-threatening post-partum haemorrhage in a large population. PLoS One. 2008; 3:e3819. PMID:
19043573.

63. Isono W, Tsutsumi R, Wada-Hiraike O, Fujimoto A, Osuga Y, Yano T, et al. Uterine artery pseudoaneurysm after cesarean section: case report and literature review. J Minim Invasive Gynecol. 2010; 17:687–691. PMID:
20656567.

64. Ko SY, Park SW, Sohn IS, Lee JY, Kwon HS, Hwang HS, et al. Interventional management for complications following caesarean section. Br J Radiol. 2011; 84:204–209. PMID:
20959367.

65. Yalamanchili S, Harvey SM, Friedman A, Shams JN, Silberzweig JE. Transarterial embolization for inferior epigastric artery injury. Vasc Endovascular Surg. 2008; 42:489–493. PMID:
19000984.

66. Deux JF, Bazot M, Le Blanche AF, Tassart M, Khalil A, Berkane N, et al. Is selective embolization of uterine arteries a safe alternative to hysterectomy in patients with postpartum hemorrhage? AJR Am J Roentgenol. 2001; 177:145–149. PMID:
11418416.

67. Sierra A, Burrel M, Sebastia C, Radosevic A, Barrufet M, Albela S, et al. Utility of multidetector CT in severe postpartum hemorrhage. Radiographics. 2012; 32:1463–1481. PMID:
22977030.

68. Lee NK, Kim S, Lee JW, Sol YL, Kim CW, Kim HS, et al. Postpartum hemorrhage: clinical and radiologic aspects. Eur J Radiol. 2010; 74:50–59. PMID:
19477095.

69. Takeda A, Koike W, Imoto S, Nakamura H. Three-dimensional computerized tomographic angiography for diagnosis and management of intractable postpartum hemorrhage. Eur J Obstet Gynecol Reprod Biol. 2014; 176:104–111. PMID:
24630300.

70. Roy-Choudhury SH, Gallacher DJ, Pilmer J, Rankin S, Fowler G, Steers J, et al. Relative threshold of detection of active arterial bleeding: in vitro comparison of MDCT and digital subtraction angiography. AJR Am J Roentgenol. 2007; 189:W238–W246. PMID:
17954618.

71. Lee NK, Kim S, Kim CW, Lee JW, Jeon UB, Suh DS. Identification of bleeding sites in patients with postpartum hemorrhage: MDCT compared with angiography. AJR Am J Roentgenol. 2010; 194:383–390. PMID:
20093600.

72. Kawamura Y, Kondoh E, Hamanishi J, Kawasaki K, Fujita K, Ueda A, et al. Treatment decision-making for post-partum hemorrhage using dynamic contrast-enhanced computed tomography. J Obstet Gynaecol Res. 2014; 40:67–74. PMID:
23937115.

73. Heaston DK, Mineau DE, Brown BJ, Miller FJ Jr. Transcatheter arterial embolization for control of persistent massive puerperal hemorrhage after bilateral surgical hypogastric artery ligation. AJR Am J Roentgenol. 1979; 133:152–154. PMID:
110056.

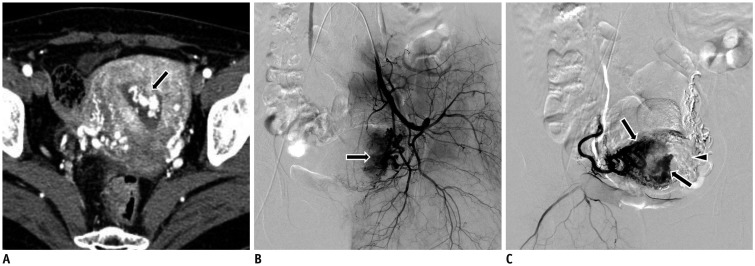
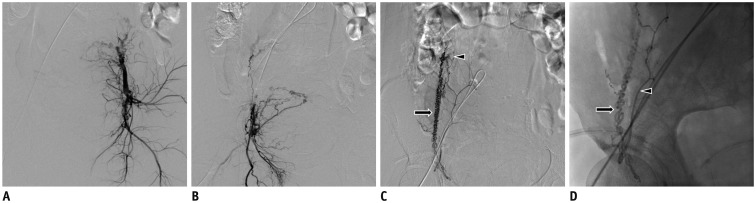




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download