Abstract
Radiofrequency ablation (RFA) has emerged as an effective loco-regional treatment modality for malignant hepatic tumors. Indeed, studies have demonstrated that RFA of early stage hepatocellular carcinomas can provide comparable overall survival to surgical resection. However, the incidence of local tumor progression (LTP) after RFA is significantly higher than that of surgical resection. Thus, to overcome this limitation, multiple electrode radiofrequency (RF) systems that use a multi-channel RF generator have been developed, and they demonstrate better efficiency in creating larger ablation zones than that using the conventional RFA with a single electrode. Furthermore, RFA with multiple electrodes can allow the “no-touch” ablation technique which may also help to reduce LTP. Another technique that would be helpful in this regard is multi-modality-ultrasound fusion imaging, which helps to not only more accurately determine the target lesion by enabling the RFA of small, poorly visible or invisible tumors, but also improve the monitoring of procedures and determine the appropriateness of the ablation margin. In addition, new energy sources, including microwave and cryoablation, have been introduced in imaging-guided tumor ablation. In this review, these recently introduced ablation techniques and the results of the most current animal and clinical studies are discussed.
During the past two decades, imaging-guided tumor ablation (IGTA) that used either chemical or thermal energy has emerged as one of the most effective loco-regional treatment modalities for small malignant hepatic tumors (1). The first tumor ablation technique to be introduced to clinical practice was the percutaneous ethanol injection (PEI), which is a chemical ablation of hepatocellular carcinomas (HCCs) (2). In the early 1990s, however, thermal ablation using radiofrequency (RF) was developed and proved its superiority to PEI in terms of better survival and local control of the disease in patients with early-stage nonsurgical HCC (3). Thereafter, other types of IGTA techniques such as microwave ablation (MWA), cryoablation, laser ablation, irreversible electroporation and high-intensity focused ultrasound (US) were developed and adopted for the treatment of malignant liver tumors (45). Among them, radiofrequency ablation (RFA) has been the most widely used method of IGTA for small malignant hepatic tumors, particularly HCC and colorectal cancer liver metastasis (CRLM), due to its safety and effectiveness as well as a reasonably good clinical outcome (67891011). Currently, it is unknown whether novel technologies will expand the clinical role of image-guided ablation and improve long-term patient outcomes with respect to RFA (4).
Regarding RFA for the management of patients with HCCs, previous studies have reported that the overall survival after RFA for early stage HCC was similar to that of surgical resection (7891112). Indeed, Cucchetti et al. (13) reported that RFA could be more cost-effective than surgical resection for patients with very early stage HCC (less than 2 cm in size), since RFA could provide excellent local tumor control. A recent practice guideline for HCC management recommends RFA as the first line treatment modality for very early stage HCC when liver transplantation is not considered (14). Previous studies demonstrated that RFA also provided acceptably good local tumor control for CRLM (615), even though the survival rate after RFA was generally poorer than that after surgical resection (10). The complication rate of RFA is also significantly lower than that of surgical resection with the reported major complication rate of RFA for HCC being generally lower than 5% (78911). Since RFA is less invasive compared to surgical resection as well as provides a reasonably good clinical outcome, currently, RFA has a major role in the management of patients with HCC as well as CRLM.
However, despite the several aforementioned merits of RFA over surgical resection, RFA has several intrinsic limitations compared to surgical resection. One of the most significant limitations of RFA is the higher rate of local tumor progression (LTP) compared to surgical resection that can occur due to an incomplete ablation at the periphery of the tumor. Regarding HCC, the reported 5-year cumulative incidence of LTP after surgical resection and RFA were less than 5% and 20–30%, respectively (7911). Tumor size and ablation margin are well-known risk factors for the development of LTP after RFA for HCC or CRLM; however, an ablation margin greater than 5–10 mm could reduce LTP rates after RFA (791116). Therefore, the creation of a larger ablation volume in a reasonable time frame without technical complexity is warranted for achieving complete ablation of HCC or CRLM. Another limitation of the percutaneous image-guided RFA is the absence of an ideal tool to guide and monitor the RFA procedure. As an example, computed tomography (CT) is not able to provide real-time guidance and has a weakness in low contrast hepatic tumors; whereas, US contain several invisible areas in the liver including the liver dome, the tip of the left lateral segment and below the ribs (17). Furthermore, an US has the limitation of effectively monitoring the procedure since gas clouds interfere with the evaluation of the relationship between the index tumor and ablation zone (181920).
There have been several efforts to improve the clinical effectiveness of the RFA procedure for the management of patients with malignant hepatic tumors by either improving the efficiency of the RF system in the creation of an ablation zone or using a more precise guiding system (2122). More recently, RFA with multiple electrodes for switching between monopolar or bipolar/multipolar modes by using a multi-channel generator or switching controller systems have been used in clinical practice, and they demonstrated that larger ablation volumes were created in a given amount of time compared to the RFA with a single electrode (23242526). In addition, the real-time US/CT and magnetic resonance (MR) image fusion technique have also demonstrated their value for expanding the feasibility of RFA and improving the therapeutic efficacy of RFA in local tumor control of HCC or CRLM (1821). In this review, the potential advantages of these novel advanced techniques in RFA of hepatic tumors is briefly discussed and the results of both animal and human studies are relayed.
Since the development of a needle-type plain RF electrode in 1994 (26), various kinds of electrodes with different designs and RF systems each with different energy control algorithms that were developed for better efficiency in creating coagulation zones have been commercially available for the thermal ablation of malignant hepatic tumors (262728). Commercially available RF electrodes can be basically classified into two types: needle and expandable types. They can also be divided into dry versus wet type. Since the electrical conductance of human tissue is low, most of the RF energy that is delivered to the tissue are deposited a few millimeters from the active portion of the RF electrodes (29). Therefore, the tissue charring from overheating (higher than 100℃) can occur in the tissue adjacent to the electrode. In order to circumvent this problem, the needle type electrodes typically use internal cooling with a pulsing algorithm; while, the umbrella type electrodes use temperature or impedance-based RF energy delivery (3031). Wet electrodes, which allow saline infusion into the target tissue, have several advantages including increased electrical conductance as well as thermal conductance and are less susceptible to tissue charring (32). However, a major disadvantage of wet electrodes is the irregular ablation shape that is created due to the inhomogeneous perfusion of saline into the tissue (32). The needle type electrodes are usually preferred for US-guided RFA due to its good visualization of the electrode tip on the US and similarity of the RF electrode placement into the index tumor of the US-guided biopsy procedure (2931). To the contrary, the expandable type electrodes are more preferred for the CT-guided RFA due to its capability for better geographical placement of tines within the index tumor. Currently, the commercially available needle type electrodes include internally cooled, wet, internally cooled-wet, bipolar, clustered and separable clustered electrodes (2628). Expandable-type electrodes include expandable and expandable-wet electrodes.
In addition, in order to improve the efficiency of the RF energy delivery per a given amount of time to the target tissue, multi-channel RF systems that use either a single generator or multiple generators have been developed for a multiple electrode RFA. Depending on the specific desired need to optimize RF energy delivery to the targeted tissue within a given amount of time, these multichannel RF systems are designed to use different electricity modes (monopolar or bipolar/multipolar) as well as different activation modes (simultaneous or switching) (31). Currently, simultaneous and switching monopolar/bipolar or multipolar modes are widely used.
Since single internally cooled electrodes can generate ablation zones that are of 2.5–3.0 cm in size, complete tumor destruction with an ablation margin that is larger than 5 mm could be achieved for tumors of approximately 1.5–2.0 cm in diameter with the placement of the electrode in the center of the index tumor (31). In contrast, for tumors between 2 cm and 3 cm in diameter, achievement of complete ablation with a sufficient ablation margin can be challenging with the use of a single electrode in a single ablation session. Therefore, multiple overlapping ablations are frequently required to create a sufficient ablation margin around the index tumor (29333435). Until now, there has been considerable difficulty in repositioning the electrode during the overlapping ablations, especially under US guidance because the echo-cloud of microbubbles develop after the first RF energy delivery, which limits the sonic window and increases the possibility of incomplete ablation (3336). Various approaches that involve a multiple-electrode RFA have been developed in order to overcome this limitation, including the simultaneous monopolar, switching monopolar, bipolar, and multipolar modes (Fig. 1).
Among the various RF energy delivery modes that use multiple electrodes, switching monopolar RFA has been the most widely used since it shares the same techniques that were developed for the conventional monopolar system with a single electrode. In switching monopolar RFA, RF energy is applied to a single electrode and then switched to another electrode (i.e., single switching monopolar mode). Previous studies regarding the use of switching monopolar RFA have reported that multiple electrodes with a switching monopolar mode RFA could provide up to a 5 cm-sized ablation zone in both animal (36) and human studies (37). Considering that the average size of the ablation zone, which can be achieved by switching monopolar RFA, is approximately 4.5–5.0 cm, switching monopolar RFA may be used for medium sized (3–5 cm) malignant hepatic tumors. In this regard, Lee et al. (25) reported that switching monopolar RFA with multiple electrodes was a safe and efficient method for the treatment of medium-sized HCCs with a technical effectiveness rate of 97% and LTP rate of 10%. Subsequently, in another study, Woo et al. (38) evaluated the mid-term results of monopolar switching RFA with multiple electrodes for the treatment of small- and medium-sized HCC. In this study, they prospectively enrolled 166 patients with a single HCC less than or equal to 5 cm in diameter, and all of the HCCs were treated by switching monopolar RFA with up to three multiple electrodes. They achieved a technical effectiveness of 99.4% and an estimated 3-year cumulative incidence of LTP of 11% (38), and 8 of their 166 patients (4.8%) experienced a major complication after RFA using multiple electrodes with switching monopolar mode, which can be considered to be similar to that of RFA using a single electrode. Another recent study demonstrated that switching monopolar RFA with separable clustered electrodes was indeed an efficient technique for the treatment of HCCs of less than 5 cm with a 100% technical effectiveness and 12.4% estimated 3-year cumulative incidence of LTP (39). Considering the results of the aforementioned studies, switching monopolar RFA with multiple electrodes may prove to be a safe and efficient treatment modality for small- and medium-sized HCCs (Fig. 2).
Since percutaneous image-guided RFA procedures are frequently performed under conscious sedation in many Asian countries, including Korea and Japan, it would be advantageous to shorten the total ablation time to less than 30 minutes by improving the RF energy delivery to the targeted tumor (404142). Despite the improved efficiency of the RF energy delivery with single switching monopolar RFA that uses multiple electrodes, frequent switching between the RF electrodes is related with an impedance rise that results in less optimal delivery of the RF energy per a given amount of time and prolongation of the procedure time. To overcome this limitation, the dual switching monopolar (DSM) RFA system has been developed. In the DSM mode, the RF energy is delivered simultaneously to two electrodes and switched between the pair of electrodes (40). Despite the electrical disturbance that can occur between the electrical currents during simultaneous RF energy delivery to multiple electrodes (Faraday effect), if the distance between the electrodes is not too close nor too far (0.5–3.0 cm), it is possible to deliver a higher amount of RF energy per a given amount of time with DSM RFA (40). Furthermore, it can allow the target tissue between the electrodes to reach a critical temperature of greater than 60℃ faster than the single switching monopolar mode, due to the thermal conductance from the two active heating zones. Thus, DSM RFA can improve the energy delivery efficiency, which creates a larger ablation zone in a given amount of time compared to the single switching monopolar mode (42). According to the results of a study performed by Yoon et al. (40), DSM RFA with three electrodes created a significantly larger ablation volume compared to the single switching monopolar RFA in a given amount of time in ex vivo bovine livers. Subsequently, Yoon et al. (41) also reported that DSM RFA created a significantly larger coagulation volume than single switching monopolar RFA in in vivo porcine livers, and the mean maximum diameter of the ablation zone that was obtained by DSM RFA with 3 internally cooled electrodes with a 3-cm active tip and 2-cm inter-electrode spacing was 4.9 cm. Considering the ex vivo and in vivo experimental results, the DSM RFA appears to have the potential to provide larger ablation zones in a given amount of time compared to the single switching monopolar mode, thereby reducing the LTP rate. Recently, Choi et al. (42) prospectively enrolled 52 patients to evaluate the feasibility and usefulness of a DSM RFA system with 3 internally cooled electrodes for the treatment of HCC. The authors compared the clinical outcome of DSM RFA to that of single switching monopolar RFA and found that DSM RFA created a significantly larger ablation volume per a given amount of time than single switching monopolar RFA (42). The estimated 2-year cumulative incidence of LTP after DSM RFA for HCC was 4.3% and seemed to be lower than the 10.1% 2-year cumulative incidence of LTP that was observed after the single switching monopolar RFA; however, statistical significance was not achieved (p = 0.15).
Currently, the majority of RF systems use the monopolar mode, in which the RF current flows from the electrode to a dispersive pad, and when the electrode is placed in the central portion of the targeted tumor, the current spreads centrifugally in a monopolar mode (31). In the bipolar mode, the RF currents flow between two electrodes; therefore, the current spreads centripetally when the electrodes are placed in a peripheral portion of the tumor (2629). Considering the difference in the sizes of the dispersive pad in the monopolar mode and passive electrode in the bipolar mode, the bipolar mode has a limitation in delivering a high amount of RF current due to high impedance; therefore, the bipolar mode requires a close distance of within 5 cm between the electrodes (43). Bipolar RFA also allows the concentration of the RF currents between the tips of the electrodes, which results in both advantages and disadvantages in the creation of a large ablation zone. The advantage of bipolar RFA is the improved efficiency of heat production per a given amount of RF energy; whereas, the disadvantage is the inherent possibility of overheating which may result in charring, a rapid rise in impedance, and insufficient RF energy delivery. Two approaches have been attempted to overcome the insufficient RF energy delivery that occurs due to the impedance rise: 1) switching bipolar/multipolar mode; and 2) saline-enhanced bipolar RFA using internally-cooled wet electrodes that allow the instillation of saline into the target tissue during RFA. In the first approach of switching bipolar or multipolar mode, when an impedance rise during RF energy delivery between one pair of electrodes was observed, switching of RF energy delivery to another pair occurs, which allows continuous RF energy delivery without a rapid impedance rise and charring (4445). The other approach is the use of saline-enhanced bipolar RFA in which there is an intratumoral injection of a saline solution during the application of the RF current that alters the tissue conductivity and thereby allows greater deposition of RF current, since the power deposition is strongly dependent on the local electrical conductivity for a given RF current (26). Although saline perfusion into tissue may have the intrinsic problem of creating irregularly shaped ablation zones due to the inhomogeneous spread of saline within the targeted tumor and surrounding tissue, this issue is much less problematic with the bipolar mode as RF current flow is limited to the area between the electrodes (32). Indeed, according to a previous experimental study, bipolar RFA using two or three internally-cooled wet electrodes was able to create more spherical shaped ablation zones than conventional switching monopolar mode RFA (46).
In addition to obtaining a larger ablation volume, the use of multiple electrodes for RFA can have another potential merit over the use of a single electrode: the use of multiple electrodes enables the “no-touch” ablation technique (Fig. 3). Traditionally, RFA of the hepatic tumor has been performed by inserting a single electrode into the central portion of the tumor. As the tumor is directly punctured during the RFA procedure, there is a potential risk of tract seeding or peritoneal seeding. The incidence of track seeding has been reported in the range of 0.3–2.8% (474849), and the prevalence of peritoneal seeding has been reported in the range of 0.2–12.5% (505152). In addition, when the electrode is inserted off-center into the tumor, the portion of the tumor that is far from the electrodemay does not adequately reach a critical temperature potentially which resultes in the development of LTP during the follow-up period. In theory, using the “no-touch” ablation technique, multiple electrodes are inserted into the outside of the target tumor rather than into the tumor, and thereby, there would be no risk of track seeding during the RFA procedure. Since the “no-touch” technique requires the insertion of multiple electrodes around the tumor, it can obtain a larger ablation zone compared to the use of a single electrode that involves a direct tumor puncture, thereby resulting in reduced LTP rates after RFA (4453). However, due to the multiple electrode insertions as well as the larger ablation zone, the possibility of major complications, such as bleeding requiring angiographic embolization and parenchymal and vascular damage, would also be increased. Regarding the clinical use of the “no-touch” ablation technique, Seror et al. (53) first reported the long-term results of “no-touch” multipolar RFA for the treatment of HCC within the Milan criteria, and demonstrated that the estimated 5-year cumulative incidence of LTP after “no-touch” multipolar RFA was 6%,which is better than the LTP of 20–30% observed with the conventional tumor puncture RFA. Subsequently, Hocquelet et al. (44) also reported that the estimated 5-year cumulative incidence of LTP after “no-touch” multipolar RFA was 7.1%, which was also significantly lower than the 28.7% 5-year LTP rate after the conventional monopolar tumor puncture RFA.The major complication rate after no-touch multipolar RFA was 4.4% in this study, which is higher than the 1.1% major complication rate that is observed after conventional monopolar tumor puncture RFA; although, the difference was not statistically significant (p = 0.054) (44). Therefore, there may be a potential risk of increased complications when using the “no-touch” ablation technique compared to the conventional monopolar tumor puncture RFA. Indeed, Lee et al. (54) recently reported that hepatic parenchymal hypo-perfusion caused by thermal vascular injury after RFA for HCC could be a significant risk factor for intrahepatic distant tumor recurrence. Therefore, accidental vascular damage, which may occur during “no-touch” RFA could be a risk of tumor recurrence after treatment, may decrease the potential benefit of lowering the LTP rate with “no-touch” RFA.
Recently, Chang et al. (2455) assessed the feasibility and optimal protocol of the “no-touch” ablation technique and evaluated both the switching monopolar mode as well as switching bipolar mode for “no-touch” ablation. In an ex vivo study that used a bovine liver, both switching monopolar and switching bipolar modes were demonstrated to be feasible for “no-touch” ablation. However, the degree of unnecessary ablation of the adjacent parenchyma was significantly lower in the switching bipolar mode compared to the switching monopolar mode. In addition, the distance between the electrode and ablation zone margin of the switching bipolar mode was significantly smaller than that of the switching monopolar mode (1.39 cm vs. 1.86 cm, p < 0.001) as RF energy can be more focused between two electrodes (55). Chang et al. (24) also assessed the incidence of adjacent organ injury during the “no-touch” ablation technique in their in vivo study using a porcine liver. They found that the frequency of adjacent organ injury after switching bipolar mode “no-touch” RFA was significantly lower than that after switching monopolar mode “no-touch” RFA (23.1% vs. 69.2%, p = 0.021). Given these ex vivo and in vivo experimental results, the switching bipolar mode may be more advantageous for “no-touch” ablation compared to the switching monopolar mode. However, to more confirmatively determine whether the “no-touch” ablation technique can significantly reduce the risk of tumor recurrence and determine which ablation mode may be more efficient and safe, further studies with a large number of patients with a prospective design are warranted.
The potential pros and cons of the use of multiple electrodes are summarized in Table 1. When multiple electrodes are used under either the switching monopolar or switching bipolar mode, a larger ablation zone can be obtained compared to the use of a single electrode; therefore, complete tumor destruction with a sufficient ablation margin for a larger tumor can be achieved. However, due to the larger ablation zone, the possibility of adjacent organ injury during the RFA procedure would also increase with the use of multiple electrodes. Indeed, as multiple needle punctures and paths are inevitably needed when multiple electrodes are used, the potential risk of bleeding would also increase. However, there has been no strong evidence of significantly increased complication rates after the use of multiple electrodes compared to the use of the single electrode. The use of multiple electrodes enables “no-touch ablation,” which can potentially reduce the risk of LTP and track seeding. When tumor size is smaller than 2 cm, both the single and multiple electrodes with switching monopolar mode can be used effectively with a high rate of technical effectiveness. However, when the tumor size is larger than 2 cm, the use of multiple electrodes with the switching monopolar/bipolar mode would be more beneficial and is now considered the reference standard ablation protocol in many centers. Since several meta-analysis studies (565758) have demonstrated that RFA combined with trans arterial chemoembolization (TACE) provides improved efficacy for intermediate-sized (3–5 cm) HCC than RFA alone, a further study, which compared RFA with multiple electrodes and the combination of RFA and TACE, is necessary.
Until now, an US has been the most commonly used imaging modality for the guidance of interventional procedures of the liver, which includes RFA of hepatic tumors due to its several merits over other imaging modalities including its real-time capability, lack of radiation exposure during the imaging study, easy accessibility, and low cost (5960). US scans of the liver are frequently obtained in oblique axial or sagittal planes rather than the true orthogonal plane; while, liver CT or MR images are routinely achieved in orthogonal planes. Therefore, during the procedure, the operators need to mentally register the reference images of the liver CT or MR examinations. Sometimes, there is difficulty in the mental registration and possibility of error during the mental registration, which can result in mis-targeting of the lesion or incomplete ablation (61). Moreover, it is well known that there may be many blind areas in the liver on the US examinations, to include the liver dome, far lateral tip of the left lobe and below the rib. The sonic window for the US examination can also be limited by the colon or omental fat that surrounds the liver. Also, with the use of surveillance tests that use biannual US for patients at high risk of developing HCC, such as those with liver cirrhosis or chronic hepatitis B viral infection, small HCCs which are good candidates for RFA, were detected more frequently than ever before (626364). However, small HCCs could be invisible on a B-mode US, especially in the background of an advanced cirrhotic liver, and RFA for this invisible small HCC might be difficult or impossible. Recently, fusion imaging, which can fuse two different imaging modalities, has been introduced to interventional procedures of the liver including RFA for hepatic tumors, which enables more accurate and precise ablation of hepatic tumors as well as small invisible hepatic tumors.
Among the various tracking methods that can be used as a fusion imaging technique, including optical, image-based, and electromagnetic (EM) tracking, EM tracking is the most widely used of the tracking methods for fusion imaging of the liver (65). In principle, there are three components in EM tracking-based fusion imaging: magnetic field generator, position sensor, and position sensor unit (21). A magnetic field generator that is located near the patient creates a magnetic field around the patient. When the position sensor, which is usually mounted on an US transducer, is moved in the magnetic field that is created by the magnetic field generator around the patient, the magnitude of the induced current within the position sensor by the magnetic field changed. With this information regarding the changes in the electrical current within the position sensor when the US transducer is moved, the position sensor unit installed in the US scanner can calculate the exact location of the position sensor; thereby, the direction and position of the US transducer can be determined (6566). The determination of the exact position and orientation of the US transducer enables a side-by-side or overlay display of real-time US images and reference CT or MR images (21). Both the internal and external markers of the patients can be used for fusion imaging of real-time US and CT/MR images. Regarding the use of external markers, the reference CT/MR images used for fusion imaging are obtained with external fiducial markers attached to the body surface near the target organ. Since the external fiducial markers contain position sensor coils and are radio-opaque on the CT images (2167), fusion imaging between the reference CT/MR images and real-time US images can be performed. However, because the reference CT images should be obtained with the attachment of external fiducial markers for the fusion imaging using external markers, this method cannot be done easily in routine clinical practice; therefore, the internal markers of patients, including anatomic landmarks, such as focal liver lesions (i.e., cysts, hemangiomas, calcifications or target lesions visible on an US), and bifurcation of the vessels, including portal or hepatic veins, have been commonly used in fusion imaging of the liver (68). Currently, most US vendors provide the fusion imaging technique between the real-time US and reference CT/MR images using the internal markers. The basic concept of fusion imaging remains similar; although, there are several differences among the different vendors.
Fusion imaging between the real-time US and reference CT/MR images by using internal markers is composed generally of three steps. The first step is uploading the reference CT/MR imaging data set, which will be fused with real-time working US images to the US machine. Then, the plane registration is conducted to select the same plane on the real-time working US and uploaded reference CT/MR images, and any plane that clearly reveals anatomic landmarks on both of the imaging data sets can be used for this step (Fig. 4). For the third step after the plane registration, the point registration can be conducted to match the two image data sets more precisely by selecting the same anatomic landmarks near the target lesion on both the real-time working US images and reference CT/MR images (Fig. 4). The point registration may repeatedly be performed to obtain optimal fusion imaging between the two modalities as well as to adjust the fusion imaging during the procedure when some mis-registrations occur that are typically due to patient respiratory motion. Since there could be a registration error between real-time working US images and reference CT/MR images even after the fusion imaging technique, it would be better to choose anatomic landmarks that are nearest to the target lesion during the point registration to reduce potential registration error. Several vendors currently provide “rotate” or “drag” functions to match real-time working US images and reference CT/MR images, which can be done on the overlay display of the two data sets instead of repeated point registration (21). After these aforementioned three steps of fusion imaging, the real-time US and reference CT/MR images are displayed side-by-side or are presented as overlaid images on US monitors that display the same plane, and move synchronously during the procedures that enabled the accurate detection of the target lesions. The time required for fusion imaging between the real-time working US and reference CT/MR images varies depending on the fusion technologies involved and the operator's experience level, but is generally approximately 1–5 minutes. According to the results of the ex vivo experimental studies assessing registration errors, there may be an approximately 3 mm error in registration between the real-time working US images and reference CT/MR images as well as in lesion targeting (6970).
Since the target lesion for RFA of hepatic tumors can be more accurately identified with the use of the imaging fusion technique compared to the use of conventional B-mode US only, the usefulness of real-time fusion imaging for RFA of hepatic tumors has been evaluated in clinical practice. Regarding lesion conspicuity, Song et al. (68) reported that the fusion imaging technique between real-time working US and reference CT/MR images could significantly improve the conspicuity of HCCs. Owing to the increased conspicuity of the target lesions, including HCCs, the feasibility of RFA for HCCs can also significantly improve after the application of fusion imaging; therefore, fusion imaging can reduce the number of RFA sessions (196869). Indeed, fusion imaging enables RFA for invisible tumors on a conventional B-mode US, as it can display reliable landmarks near the target tumor that is visible on the reference CT/MR and real-time working US images, and operators can more confidently perform RFA for invisible tumors (2168). Considering that the detectability of HCCs on a conventional B-mode US primarily depends on the tumor size (6071), fusion imaging between the real-time working US and reference CT/MR images could be much more beneficial for small HCCs that are less than 2 cm in size, which may be invisible on a conventional US, especially in the background of advanced cirrhosis, compared to HCCs that are larger than 2 cm in size (20). With the promising results of these initial studies regarding the use of fusion imaging in the performance of RFA for hepatic tumors, Ahn et al. (18) recently reported the results of their prospective study that included 216 patients with 243 HCCs treated by RFA under fusion imaging guidance. They found that after applying the fusion imaging technique, tumor visibility and technical feasibility of the RFA procedures significantly improved. In addition, the technical effectiveness of RFA for invisible tumors on the conventional B-mode US was similar to that for visible tumors with the use of fusion imaging (18) (Fig. 5). Considering the results of these aforementioned studies, fusion imaging can be confidently used for RFA of hepatic tumors, which significantly increases the technical effectiveness of RFA procedures, especially for small invisible tumors on a conventional B-mode US (Fig. 5). Furthermore, the combined guidance of a contrast-enhanced US and fusion imaging in RFA can improve the operator's confidence for inconspicuous HCC on a B-mode US (72).
In addition to increasing the conspicuity of the targeted tumors and feasibility of RFA procedures, fusion imaging can also help monitor RFA procedures. Potential complications and adjacent organ injury that arises after RFA might be avoided since fusion imaging can show the relationship between the ablation zone and vital structures, including the bile duct and large portal vein as well as adjacent organs, better than a B-mode US. These potential merits of fusion imaging in the monitoring of RFA procedures can be more pronounced with the use of multiple electrodes. When multiple electrodes with switching monopolar/bipolar mode are used, larger ablation zones of up to 5 cm in size can be obtained; therefore, RFA for larger hepatic tumors can be possible. However, at the same time, larger ablation zones that are obtained by the use of multiple electrodes with switching monopolar/bipolar mode can also cause collateral thermal damage to adjacent organs or vital structures. Because the fusion imaging technique can also display the tumor itself as well as adjacent organs and vital structures, collateral thermal damage to adjacent structures could be reduced. Last but not least, the fusion imaging technique can also be used to determine the appropriateness of the ablation margins since it can precisely show the exact location of the target tumor on the real-time working US images referenced by the synchronously-moved CT/MR images, which reveal the accurate location of the target tumor. When the target tumors on the real-time working US images after imaging fusion entirely covers the echo-cloud of micro-bubbles with a sufficient margin after RF energy delivery, successful ablation without any residual tumors is expected.
Even after the point registration is conducted by identifying the same anatomic landmarks near the target lesion on both the real-time working US and reference CT/MR images to obtain accurate registration, there may be registration errors in the fusion imaging technique. One of the causes of this error is the different acquisition statuses between the reference CT/MR and real-time working US images. The reference CT/MR images are usually obtained during breath-hold, and therefore, can be regarded as static images. However, the real-time working US images are usually scanned during free-breathing, and thus can be affected by the deformation of the liver during respiration or patient movement. It is well-known that the liver moves three-dimensionally during the patient respiratory cycle, and that the liver volume can also change during the respiration to some degree. Since most commercially available fusion imaging systems use the rigid registration algorithm, the potential difference between the static reference CT/MR and three-dimensionally deformed real-time working US images may not always be compensated (66). To reduce this potential registration error, it would be better to use the same image data set that was obtained during the same respiratory cycle. For example, since MR images are usually obtained during the expiratory phase, the real-time working US images that are scanned during the expiratory phase would be better to use for the fusion imaging when the MR images are used as a reference (21).
The peripherally-located target tumors can be another limitation of fusion imaging-guided RFA. According to a recent study, mis-targeting occurred in 1.3% of patients (7/551) with HCCs treated by RFA even under the guidance of fusion imaging (73), and the majority of mis-targeted HCCs were less than 1.5 cm in size and located in the peripheral portion of the liver. Peripherally-located target lesions would be more prone to registration error in the fusion imaging technique because it might be difficult to use large hepatic vessels as anatomic landmarks to align the peripherally-located target lesion on both the real-time working US and reference CT/MR images, and the distance between the anatomic landmarks and target lesions could be long, and result in increased registration error. Indeed, liver deformation and displacement during the respiratory cycle might be more pronounced in the peripheral portion of the liver compared to the central portion (73). Therefore, contrast-enhanced US can be helpful for the challenging case of RFA for small, peripherally located target tumors, and contrast-enhanced US combined with fusion imaging can further decrease the possibility of mis-targeting (21). Finally, because the hepatitis B virus (HBV) infection causes macro-nodular cirrhosis that can contain numerous pseudo-lesions that possibly mimic the targeted tumor on US examinations (61), caution should be taken for RFA of HCC in patients with HBV-related cirrhosis even under fusion imaging guidance.
In addition to RF energy, various energy sources, including microwave, laser, high intensity-focused US and cryoablation, have been used for IGTA (74). Among them, MWA has gained popularity due to its several merits of MWA over RFA and already replacing RFA in the management of liver malignancies at several institutions, particularly in western countries. MWA can induce a higher intra-tumoral temperature within a short ablation time, which can create a larger ablation zone than RFA and also have less heat sink effect compared to RFA (7576). Therefore, it is expected that MWA will provide better local tumor control for liver malignancies than RFA. However, the reported LTP rate of MWA for HCC has ranged from 3.9% to 42.0%; yet, it seems to be comparable to that of RFA (777879). In addition, according to the recent studies, the therapeutic efficacy and safety of MWA for HCC was comparable to that of RFA regarding the LTP rate, progression-free survival, overall survival as well as major complication rates (767980).
Cryoablation has emerged as another energy source for IGTA. Because cryoablation uses cold instead of heat, there could be potential advantages in cryoablation compared to thermal ablation such as RFA or MWA, which includes easier in situ monitoring of the ablation zone and better correlation of the ablation area on imaging and pathology. The edge of the iceball that is created during the cryoablation is very visible on the CT and MR images. However, on the US, while the anterior edge of the iceball is also well visualized, it is difficult to assess the posterior edge of the iceball due to the posterior shadowing (81). In addition, the extent of tissue necrosis is well correlated with the size of the iceball; although, the size of the iceball is slightly larger than that of the necrosis (81). Therefore, more accurate monitoring of the ablation procedure enables both better local tumor control and lower complication rates. Another merit of cryoablation over the other thermal ablation techniques is less pain during and after the procedure. Thermal ablation, including RFA and MWA, can cause severe pain that might interfere with the ablation procedures. To the contrary, cryoablation is less painful, and patients usually well tolerate the procedure. However, the downside of cryoablation can be the prolonged ablation time. With recent advances in the ablation devices, the ablation time in RFA is usually less than 20 minutes even in medium-sized HCC, and the ablation time in MWA is even less than RFA. However, since cryoablation requires multiple cycles of a freezing and thawing process to obtain sufficient tissue destruction, the ablation time in cryoablation is usually more than 30 minutes. In addition, cryoablation generally needs the insertion of multiple probes to obtain a larger iceball through the synergistic effect of each probe. Regarding the therapeutic efficacy of cryoablation for HCC, Wang et al. (82) recently reported the result of their prospective randomized controlled trial comparing the therapeutic efficacy of cryoablation for HCC to that of RFA, and the LTP rate after ablation was significantly lower in the cryoablation group than the RFA group even though there was no significant difference in the overall survival, progression-free survival and major complication rate. This significantly lower LTP rate of cryoablation might be explained by the more precise monitoring of the ablation procedure of cryoablation compared to RFA. However, for the generalization of this result, additional studies with a larger number of patients are warranted. Currently, it is unknown whether novel technologies will expand the clinical role of IGTA and improve long-term patient outcomes with respect to RFA (4).
During the past two decades, RFA has emerged as an effective loco-regional treatment modality for malignant hepatic tumors, especially for HCCs and CRLMs. However, the significantly higher rate of LTP compared to surgical resection has been one of the main limitations of RFA for hepatic tumors, especially for tumors larger than 3 cm in size. Since the use of multiple electrodes with various RF energy delivery methods can provide larger ablation zones of up to 5 cm, RFA for small- and medium-sized tumors of 3–5 cm in size would be possible with the use of multiple electrodes. However, at the same time, to obtain larger ablation zones for medium-sized hepatic tumors using multiple electrode RFA techniques, the possibility of collateral thermal damage to adjacent organ/structure would also increase. Therefore, more cautious and meticulous planning prior to RFA as well as monitoring during the procedure are warranted. The recently introduced fusion imaging technique between the real-time US and reference CT/MR images enable more accurate determination and targeting of hepatic tumors, which increases the technical effectiveness of RFA for hepatic tumors, especially among small, inconspicuous tumor displayed on a conventional B-mode US. Furthermore, the fusion imaging technique can also help monitor the ablation procedures as well as determine the appropriateness of the ablation margin during the procedures, since it can provide both the accurate location of the target lesions and adjacent vital structures/organs on the real-time working US images as referenced by the synchronously moving CT/MR images, which can accurately display the target tumors as well as adjacent structures/organs. Therefore, the multimodality fusion imaging system and multiple electrode RFA approaches could synergistically be used to improve the local tumor control rate of RFA. Operators should be familiar with these recently introduced techniques to obtain the most optimal outcome for RFA of hepatic tumors.
Figures and Tables
 | Fig. 1Diagram showing use of three electrodes with various RF energy delivery methods.
A. Single switching monopolar mode: RF energy is delivered to one of three electrodes at given time, and switched to adjacent electrode. Ground pad serves as passive electrode. B. Dual switching monopolar mode: RF energy is simultaneously delivered to pair of electrodes among three inserted electrodes at given time, and switched to pair of electrodes. Ground pad serves as passive electrode. C. Switching bipolar mode: pair of electrodes is activated, and then switched to another pair of electrodes: one electrode serves as active electrode and other as passive electrode. Green circle: inserted electrode; red circle: active electrode; blue circle and blue bar: passive electrode; and light orange or eclipse: ablation zone. RF = radiofrequency
|
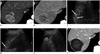 | Fig. 2Switching monopolar RFA with multiple electrodes for medium-sized HCCs.
A. Contrast-enhanced arterial phase transverse CT image displays 4.2 cm enhancing mass in segment VII of liver. B. Portal venous phase transverse CT image shows wash-out of tumor, which indicated HCC. C, D. Under US guidance, three electrodes with 3-cm active tip (arrows) are placed across index tumor. E. After RF energy delivery with switching monopolar mode, echo-cloud of microbubbles are generated, encompassing index tumor. F. One-month follow-up contrast-enhanced portal venous phase transverse CT image displays complete ablation of index tumor without evidence of viable residual tumor. CT = computed tomography, HCCs = hepatocellular carcinomas, RFA = radiofrequency ablation, US = ultrasound
|
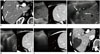 | Fig. 3RFA for HCC using multiple electrodes with “no-touch” technique under fusion imaging guidance.
A. Contrast-enhanced arterial phase transverse CT image displays 1.5-cm enhancing nodular lesion (arrow) in segment VI of liver, which suggests HCC. B. Fusion imaging technique between real-time working US and reference arterial phase CT images clearly display low echoic target lesion on US image. C. Under fusion imaging guidance, two electrodes (arrows) are inserted outside of target tumor (*). Tumor itself is not violated during electrode insertion. D. After RF energy delivery, echo-cloud of micro-bubbles is created around target tumor. E. Echo-cloud of microbubbles completely covers target tumor, which suggests successful ablation of target tumor. F. Immediate follow-up contrast-enhanced portal venous phase transverse CT image demonstrates complete destruction of target tumor with sufficient ablation margin.
|
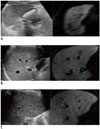 | Fig. 4Process of fusion imaging between real-time US and reference MR images.
A. Plane registration: after loading of reference MR images to US equipment that implemented fusion imaging technique; plane registration was done. In this case, sagittal plane was used for plane registration. B. Point registration: after plane registration, point registration was performed by selecting same anatomic landmark (proximal right portal vein in this case) on both real-time working US and reference MR images. C. Target tumor marking: after point registration, targeted tumor was marked on reference MR image. Location of targeted tumor on real-time working US was immediately visualized in corresponding location. MR = magnetic resonance
|
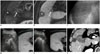 | Fig. 5RFA of small invisible tumor on conventional B-mode US under fusion imaging guidance.
A. Subtraction image from arterial phase gadoxetic acid-enhanced MR image to precontrast MR image demonstrates 1.2-cm arterial enhancing nodular lesion in segment V subcapsular portion of liver (arrow). There is non-enhancing area (*) due to previous ablation therapy. B. Hepatobiliary phase image displays low signal intensity within tumor (arrow), which suggests HCC. Previous ablation zone also exhibited low signal intensity (*). C. On conventional B-mode US image, there is no focal lesion corresponding to HCC that is visible on gadoxetic acid-enhanced MR. D. Under fusion imaging guidance between real-time working US and reference hepatobiliary phase MR images, location of index tumor is determined, and electrode is inserted. E. After RF energy delivery, echo-cloud of micro-bubbles is created and encompasses index tumor. F. Immediate follow-up contrast-enhanced portal venous phase transverse CT image displays complete destruction of target tumor with sufficient ablation margin.
|
Table 1
Potential Pros and Cons of Using Multiple Electrodes in Radiofrequency Ablation Procedure

References
1. Ahmed M. Technology Assessment Committee of the Society of Interventional Radiology. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014; 25:1706–1708.

2. Shiina S, Tateishi R, Imamura M, Teratani T, Koike Y, Sato S, et al. Percutaneous ethanol injection for hepatocellular carcinoma: 20-year outcome and prognostic factors. Liver Int. 2012; 32:1434–1442.

3. Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009; 9:31.

4. Lencioni R, de Baere T, Martin RC, Nutting CW, Narayanan G. Image-guided ablation of malignant liver tumors: recommendations for clinical validation of novel thermal and non-thermal technologies - a western perspective. Liver Cancer. 2015; 4:208–214.

5. Habib A, Desai K, Hickey R, Thornburg B, Lewandowski R, Salem R. Locoregional therapy of hepatocellular carcinoma. Clin Liver Dis. 2015; 19:401–420.

6. Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001; 221:159–166.

7. N'Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 western patients with cirrhosis. Hepatology. 2009; 50:1475–1483.
8. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012; 107:569–577. quiz 578.

9. Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013; 58:89–97.

10. Minami Y, Kudo M. Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver. 2013; 7:1–6.

11. Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014; 270:900–909.

12. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006; 243:321–328.

13. Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013; 59:300–307.

15. Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes--a 10-year experience at a single center. Radiology. 2016; 278:601–611.

16. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010; 195:758–765.
17. Kang TW, Lee MW, Song KD, Rhim H, Lim HK, Kang W, et al. Ultrasound-guided radiofrequency ablation using a new electrode with an electromagnetic position sensor for hepatic tumors difficult to place an electrode: a preliminary clinical study. Cardiovasc Intervent Radiol. 2017; 40:1891–1898.

18. Ahn SJ, Lee JM, Lee DH, Lee SM, Yoon JH, Kim YJ, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017; 66:347–354.

19. Lee MW, Rhim H, Cha DI, Kim YJ, Choi D, Kim YS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol. 2012; 198:1438–1444.

20. Lee MW, Rhim H, Cha DI, Kim YJ, Lim HK. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1–3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013; 24:958–965.

21. Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014; 33:227–239.

22. Kang TW, Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer. 2015; 4:176–187.

23. Brace CL, Sampson LA, Hinshaw JL, Sandhu N, Lee FT Jr. Radiofrequency ablation: simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J Vasc Interv Radiol. 2009; 20:118–124.

24. Chang W, Lee JM, Yoon JH, Lee DH, Lee SM, Lee KB, et al. No-touch radiofrequency ablation using multiple electrodes: an in vivo comparison study of switching monopolar versus switching bipolar modes in porcine livers. PLoS One. 2017; 12:e0176350.

25. Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012; 13:34–43.

26. Mulier S, Miao Y, Mulier P, Dupas B, Pereira P, de Baere T, et al. Electrodes and multiple electrode systems for radiofrequency ablation: a proposal for updated terminology. Eur Radiol. 2005; 15:798–808.

27. Denys AL, De Baere T, Kuoch V, Dupas B, Chevallier P, Madoff DC, et al. Radio-frequency tissue ablation of the liver: in vivo and ex vivo experiments with four different systems. Eur Radiol. 2003; 13:2346–2352.

28. Pereira PL, Trübenbach J, Schenk M, Subke J, Kroeber S, Schaefer I, et al. Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology. 2004; 232:482–490.

29. Ni Y, Mulier S, Miao Y, Michel L, Marchal G. A review of the general aspects of radiofrequency ablation. Abdom Imaging. 2005; 30:381–400.

30. Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities--part I. J Vasc Interv Radiol. 2001; 12:1021–1032.
31. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001; 13:129–147.

32. Lee JM, Han JK, Kim SH, Lee JY, Kim DJ, Lee MW, et al. Saline-enhanced hepatic radiofrequency ablation using a perfused-cooled electrode: comparison of dual probe bipolar mode with monopolar and single probe bipolar modes. Korean J Radiol. 2004; 5:121–127.

33. Dodd GD 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001; 177:777–782.
34. Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001; 12:1135–1148.

35. Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004; 232:260–271.

36. Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007; 42:163–171.
37. Laeseke PF, Frey TM, Brace CL, Sampson LA, Winter TC 3rd, Ketzler JR, et al. Multiple-electrode radiofrequency ablation of hepatic malignancies: initial clinical experience. AJR Am J Roentgenol. 2007; 188:1485–1494.

38. Woo S, Lee JM, Yoon JH, Joo I, Kim SH, Lee JY, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology. 2013; 268:589–600.

39. Choi JW, Lee JM, Lee DH, Yoon JH, Suh KS, Yoon JH, et al. Switching monopolar radiofrequency ablation using a separable cluster electrode in patients with hepatocellular carcinoma: a prospective study. PLoS One. 2016; 11:e0161980.

40. Yoon JH, Lee JM, Han JK, Choi BI. Dual switching monopolar radiofrequency ablation using a separable clustered electrode: comparison with consecutive and switching monopolar modes in ex vivo bovine livers. Korean J Radiol. 2013; 14:403–411.
41. Yoon JH, Lee JM, Hwang EJ, Hwang IP, Baek J, Han JK, et al. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol. 2014; 15:235–244.
42. Choi TW, Lee JM, Lee DH, Lee JH, Yu SJ, Kim YJ, et al. Percutaneous dual-switching monopolar radiofrequency ablation using a separable clustered electrode: a preliminary study. Korean J Radiol. 2017; 18:799–808.

43. Lee JM, Han JK, Kim SH, Lee JY, Shin KS, Choi BI. An ex-vivo experimental study on optimization of bipolar radiofrequency liver ablation using perfusion-cooled electrodes. Acta Radiol. 2005; 46:443–451.

44. Hocquelet A, Aubé C, Rode A, Cartier V, Sutter O, Manichon AF, et al. Comparison of no-touch multi-bipolar vs. monopolar radiofrequency ablation for small HCC. J Hepatol. 2017; 66:67–74.

45. Seror O, Sutter O. RE: should we use a monopolar or bipolar mode for performing no-touch radiofrequency ablation of liver tumors? Clinical practice might have already resolved the matter once and for all. Korean J Radiol. 2017; 18:749–752.

46. Yoon JH, Lee JM, Woo S, Hwang EJ, Hwang I, Choi W, et al. Switching bipolar hepatic radiofrequency ablation using internally cooled wet electrodes: comparison with consecutive monopolar and switching monopolar modes. Br J Radiol. 2015; 88:20140468.

47. Espinoza S, Briggs P, Duret JS, Lapeyre M, de Baère T. Radiofrequency ablation of needle tract seeding in hepatocellular carcinoma. J Vasc Interv Radiol. 2005; 16:743–746.

48. Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005; 16:485–491.

49. Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007; 33:437–444.
50. Llovet JM, Vilana R, Brü C, Bianchi L, Salmeron JM, Boix L, et al. Barcelona Clínic Liver Cancer (BCLC) Group. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001; 33:1124–1129.

51. Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002; 89:1206–1222.

52. Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. First-line radiofrequency ablation with or without artificial ascites for hepatocellular carcinomas in a subcapsular location: local control rate and risk of peritoneal seeding at long-term follow-up. Clin Radiol. 2013; 68:e641–e651.

53. Seror O, N’Kontchou G, Nault JC, Rabahi Y, Nahon P, Ganne-Carrié N, et al. Hepatocellular carcinoma within Milan criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology. 2016; 280:611–621.

54. Lee DH, Lee JM, Yoon JH, Kim YJ, Han JK. Thermal injury-induced hepatic parenchymal hypoperfusion: risk of hepatocellular carcinoma recurrence after radiofrequency ablation. Radiology. 2017; 282:880–891.

55. Chang W, Lee JM, Lee SM, Han JK. No-touch radiofrequency ablation: a comparison of switching bipolar and switching monopolar ablation in ex vivo bovine liver. Korean J Radiol. 2017; 18:279–288.
56. Wang X, Hu Y, Ren M, Lu X, Lu G, He S. Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: a time-to-event meta-analysis. Korean J Radiol. 2016; 17:93–102.

57. Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013; 19:3872–3882.

58. Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013; 25:187–194.
59. Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005; 234:961–967.

60. Rhim H, Lee MH, Kim YS, Choi D, Lee WJ, Lim HK. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR Am J Roentgenol. 2008; 190:1324–1330.

61. Lee MW, Lim HK, Kim YJ, Choi D, Kim YS, Lee WJ, et al. Percutaneous sonographically guided radio frequency ablation of hepatocellular carcinoma: causes of mistargeting and factors affecting the feasibility of a second ablation session. J Ultrasound Med. 2011; 30:607–615.
62. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.

63. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943.
64. Lee DH, Lee JM, Lee JY, Kim SH, Kim JH, Yoon JH, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: risk of HCC recurrence after radiofrequency ablation. J Hepatol. 2015; 62:1122–1130.

65. Maybody M, Stevenson C, Solomon SB. Overview of navigation systems in image-guided interventions. Tech Vasc Interv Radiol. 2013; 16:136–143.

66. Ewertsen C, Sáftoiu A, Gruionu LG, Karstrup S, Nielsen MB. Real-time image fusion involving diagnostic ultrasound. AJR Am J Roentgenol. 2013; 200:W249–W255.

67. Abi-Jaoudeh N, Kruecker J, Kadoury S, Kobeiter H, Venkatesan AM, Levy E, et al. Multimodality image fusion-guided procedures: technique, accuracy, and applications. Cardiovasc Intervent Radiol. 2012; 35:986–998.

68. Song KD, Lee MW, Rhim H, Cha DI, Chong Y, Lim HK. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol. 2013; 201:1141–1147.

69. Crocetti L, Lencioni R, Debeni S, See TC, Pina CD, Bartolozzi C. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol. 2008; 43:33–39.
70. Lee JY, Choi BI, Chung YE, Kim MW, Kim SH, Han JK. Clinical value of CT/MR-US fusion imaging for radiofrequency ablation of hepatic nodules. Eur J Radiol. 2012; 81:2281–2289.

71. Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010; 194:W396–W400.

72. Minami T, Minami Y, Chishina H, Arizumi T, Takita M, Kitai S, et al. Combination guidance of contrast-enhanced US and fusion imaging in radiofrequency ablation for hepatocellular carcinoma with poor conspicuity on contrast-enhanced US/fusion imaging. Oncology. 2014; 87:Suppl 1. 55–62.

73. Lim S, Lee MW, Rhim H, Cha DI, Kang TW, Min JH, et al. Mistargeting after fusion imaging-guided percutaneous radiofrequency ablation of hepatocellular carcinomas. J Vasc Interv Radiol. 2014; 25:307–314.

74. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014; 273:241–260.

75. Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010; 17:171–178.

76. Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013; 82:1379–1384.

77. Lee KF, Hui JW, Cheung YS, Wong JS, Chong CN, Wong J, et al. Surgical ablation of hepatocellular carcinoma with 2.45-GHz microwave: a critical appraisal of treatment outcomes. Hong Kong Med J. 2012; 18:85–91.
78. Abdelaziz A, Elbaz T, Shousha HI, Mahmoud S, Ibrahim M, Abdelmaksoud A, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014; 28:3429–3434.

79. Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015; 7:1054–1063.
80. Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005; 40:1054–1060.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download