Abstract
Purpose
To investigate the relationships between myopia and the three parameters of intraocular pressure (IOP), estimated cerebrospinal fluid pressure (CSFP), and the trans-lamina cribrosa pressure difference (TLCPD).
Methods
A total of 6,933 adults (≥19 years of age) who participated in the Korea National Health and Nutrition Examination Survey (2008–2012). These subjects were divided into two groups: young age group (19–49 years of age) and old age group (≥50 years of age). The estimated CSFP was calculated as CSFP (mmHg) = 0.44 body mass index (kg/m2) + 0.16 diastolic blood pressure (mmHg) – 0.18 age (years) – 1.91. The TLCPD was calculated by subtracting the CSFP from the IOP.
Results
The mean estimated CSFP in the total population was 13.7 ± 0.1 mmHg (young, 14.2 ± 0.1 mmHg; old, 11.5 ± 0.1; p < 0.01), the mean IOP in the total population was 14.0 ± 0.1 mmHg (young, 14.0 ± 0.1 mmHg; old, 14.1 ± 0.1; p = 0.724), and the mean TLCPD in the total population was 0.7 ± 0.1 mmHg (young, 0.3 ± 0.1 mmHg; old, 3.0 ± 0.2; p < 0.001). After adjusting for confounding factors, multivariate linear regression analyses revealed significant positive associations between the degree of myopia and the estimated CSFP (p < 0.001; β, 0.12; spherical equivalent [SE], 0.03), as well as IOP (p < 0.001; β, 0.29; SE, 0.05). As a result, a higher TLCPD also showed a significant association with more myopic refractive error (p=0.002; β, 0.18; SE, 0.06). In subgroup analyses, a similar association was shown only in the young age group (estimated CSFP, p < 0.001; β, 0.12; SE, 0.03; IOP, p < 0.001; β, 0.28; SE, 0.05; TLCPD, p = 0.005; β, 0.17; SE: 0.06), while the old age group did not show a significant association between TLCPD and the degree of myopia (p = 0.274; β, 0.18; SE, 0.16).
Go to : 
References
1. Crawford Downs J, Roberts MD, Sigal IA. Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active abdominal remodeling as a mechanism. Exp Eye Res. 2011; 93:133–40.
2. Wu HM, Seet B, Yap EP, et al. Does education explain ethnic abdominals in myopia prevalence? A population-based study of young adult males in Singapore. Optom Vis Sci. 2001; 78:234–9.
3. Tomlinson A, Phillips CI. Ratio of optic cup to optic disc. In relation to axial length of eyeball and refraction. Br J Ophthalmol. 1969; 53:765–8.

4. Bellezza AJ, Hart RT, Burgoyne CF. The optic nerve head as a abdominal structure: initial finite element modeling. Invest Ophthalmol Vis Sci. 2000; 41:2991–3000.
5. Chihara E, Sawada A. Atypical nerve fiber layer defects in high myopes with high-tension glaucoma. Arch Ophthalmol. 1990; 108:228–32.

6. Dichtl A, Jonas JB, Naumann GO. Histomorphometry of the optic disc in highly myopic eyes with absolute secondary angle closure glaucoma. Br J Ophthalmol. 1998; 82:286–9.

7. Cahane M, Bartov E. Axial length and scleral thickness effect on susceptibility to glaucomatous damage: a theoretical model im-plementing Laplace's law. Ophthalmic Res. 1992; 24:280–4.

8. Quigley HA. Reappraisal of the mechanisms of glaucomatous abdominal nerve damage. Eye (Lond). 1987; 1(Pt 2):318–22.
9. Avetisov ES, Savitskaya NF. Some features of ocular abdominal in myopia. Ann Ophthalmol. 1977; 9:1261–4.
10. Shih YF, Horng IH, Yang CH, et al. Ocular pulse amplitude in myopia. J Ocul Pharmacol. 1991; 7:83–7.

11. To'mey KF, Faris BM, Jalkh AE, Nasr AM. Ocular pulse in high myopia: a study of 40 eyes. Ann Ophthalmol. 1981; 13:569–71.
13. Lütjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1981; 21:563–73.
14. Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera of high myopia. An electron microscopic study. Arch Ophthalmol. 1979; 97:912–5.
15. Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and envi-ronment in refractive error: the Twin Eye Study. Invest Ophthalmol Vis Sci. 2001; 42:1232–6.
16. Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997; 275:668–70.

17. Mutti DO, Zadnik K, Adams AJ. Myopia. The nature versus nurture debate goes on. Invest Ophthalmol Vis Sci. 1996; 37:952–7.
18. Morgan WH, Yu DY, Cooper RL, et al. The influence of abdominal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest Ophthalmol Vis Sci. 1995; 36:1163–72.
19. Ren R, Jonas JB, Tian G, et al. Cerebrospinal fluid pressure in abdominal: a prospective study. Ophthalmology. 2010; 117:259–66.
20. Marek B, Harris A, Kanakamedala P, et al. Cerebrospinal fluid pressure and glaucoma: regulation of trans-lamina cribrosa pressure. Br J Ophthalmol. 2014; 98:721–5.

21. Xie X, Zhang X, Fu J, et al. Noninvasive intracranial pressure abdominal by orbital subarachnoid space measurement: the Beijing Intracranial and Intraocular Pressure (iCOP) Study. Crit Care. 2013; 17:R162.
22. Choi JA, Han K, Park YM, Park CK. Age-related association of abdominal error with intraocular pressure in the Korea National Health and Nutrition Examination Survey. PLoS One. 2014; 9:e111879.
23. Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship abdominal glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999; 106:2010–5.
24. Xu L, Wang Y, Wang S, et al. High myopia and glaucoma abdominal the Beijing Eye Study. Ophthalmology. 2007; 114:216–20.
25. Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic abdominal and meta-analysis. Ophthalmology. 2011; 118:1989–94.e2.
26. Shim SH, Sung KR, Kim JM, et al. The Prevalence of Open-Angle Glaucoma by Age in Myopia: The Korea National Health and Nutrition Examination Survey. Curr Eye Res. 2017; 42:65–71.

27. Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid abdominal is decreased in primary open-angle glaucoma. Ophthalmology. 2008; 115:763–8.
28. Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension abdominal, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci. 2008; 49:5412–8.
29. Lee SH, Kwak SW, Kang EM, et al. Estimated Trans-Lamina Cribrosa Pressure Differences in Low-Teen and High-Teen Intraocular Pressure Normal Tension Glaucoma: The Korean National Health and Nutrition Examination Survey. PLoS One. 2016; 11:e0148412.

30. Killer HE, Miller NR, Flammer J, et al. Cerebrospinal fluid abdominal in the optic nerve in normal-tension glaucoma. Br J Ophthalmol. 2012; 96:544–8.
31. Linden C, Qvarlander S, Johannesson G, et al. Normal-tension glaucoma has normal intracranial pressure: a prospective study of intracranial pressure and intraocular pressure in different body positions. Ophthalmology. 2018; 125:361–8.
32. Fan H, Ma H, Gao R, et al. Associated factors for visibility and width of retrobulbar subarachnoid space on swept-source optical coherence tomography in high myopia. Sci Rep. 2016; 6:36723.

34. Fleischman D, Bicket AK, Stinnett SS, et al. Analysis of abdominal fluid pressure estimation using formulae derived from clinical data. Invest Ophthalmol Vis Sci. 2016; 57:5625–30.
35. Jonas JB, Nangia V, Matin A, et al. Intraocular pressure and abdominal factors: the Central India Eye and Medical Study. J Glaucoma. 2011; 20:405–9.
36. Kim YK, Tumurbaatar U, Ohn YH, et al. Cerebrospinal fluid pressure and trans-lamina cribrosa pressure difference in open-angle abdominal: KNHANES V. J Korean Ophthalmol Soc. 2016; 57:1392–9.
Go to : 
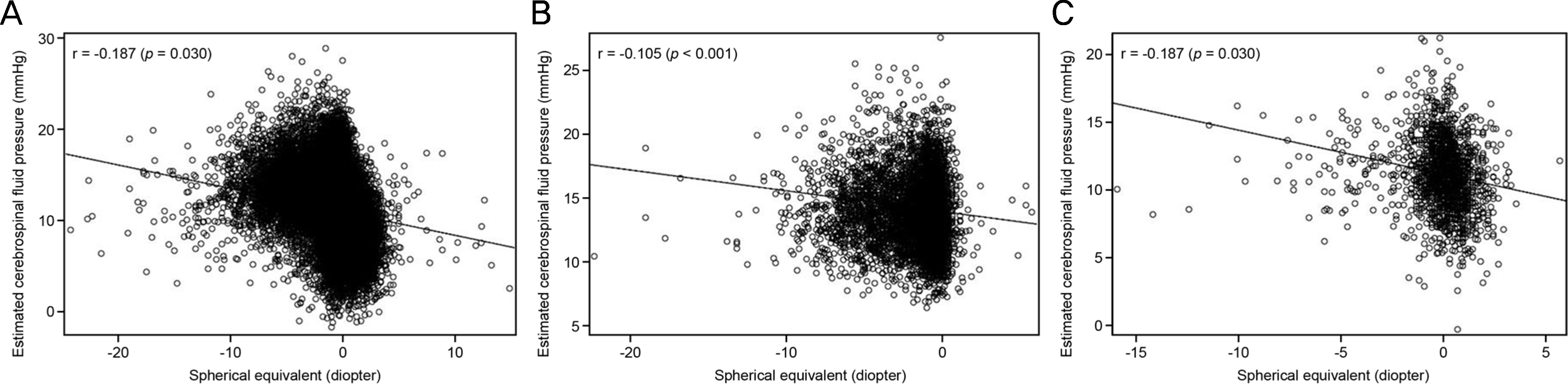 | Figure 1.Spherical equivalent and estimated cerebrospinal fluid pressure. Association between estimated cerebrospinal fluid pressure and spherical equivalent in the total study population (A), young (B) and old age group (C). |
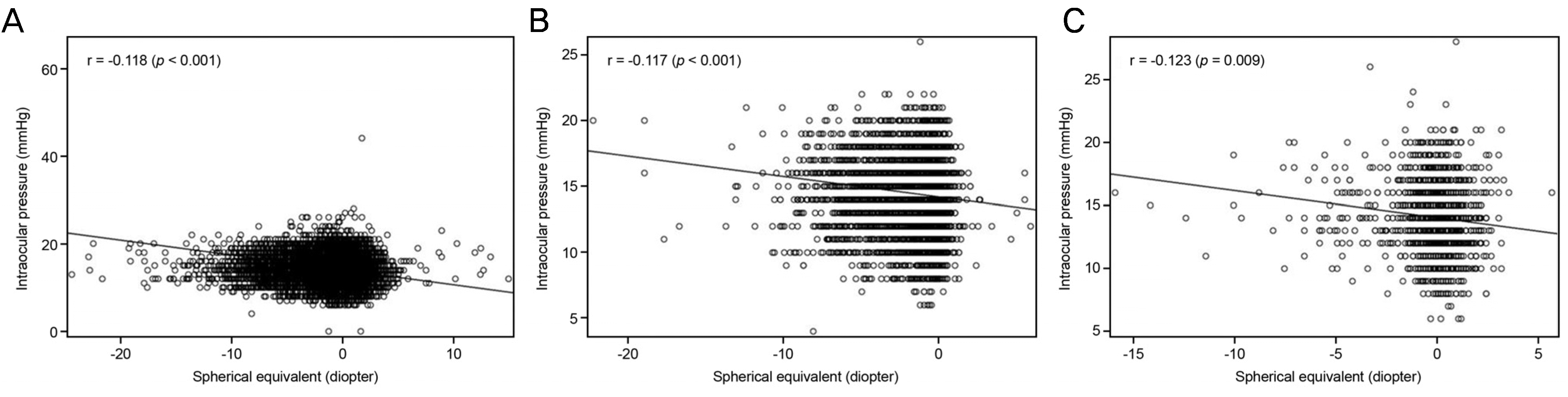 | Figure 2.Spherical equivalent and intraocular pressure. Association between Intraocular pressure and spherical equivalent in the total study population (A), young (B), and old age group (C). |
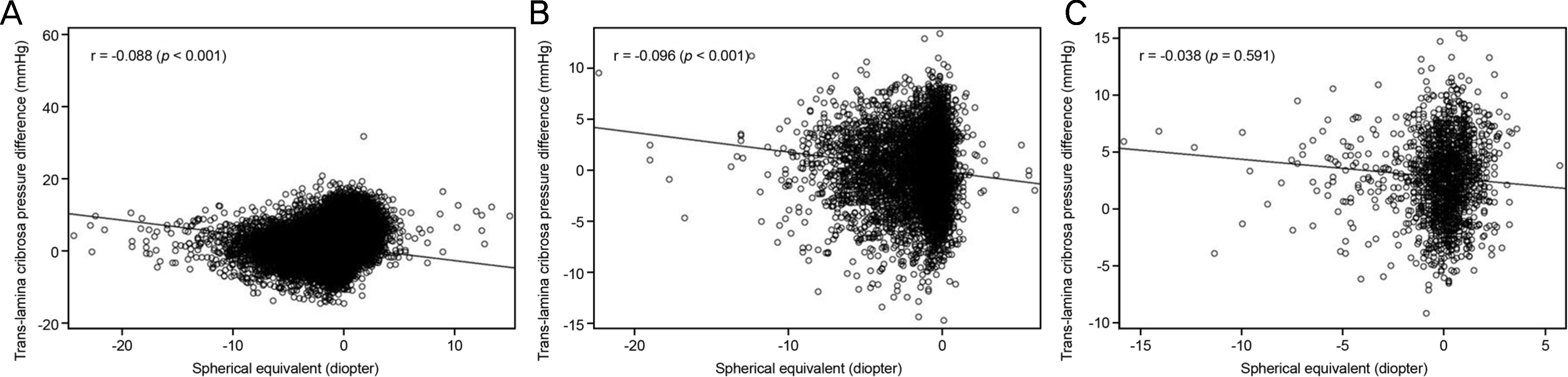 | Figure 3.Spherical equivalent and Trans-lamina cribrosa pressure difference. Association between Trans-lamina cribrosa pressure difference and spherical equivalent in the total study population (A), young (B), and old age group (C). |
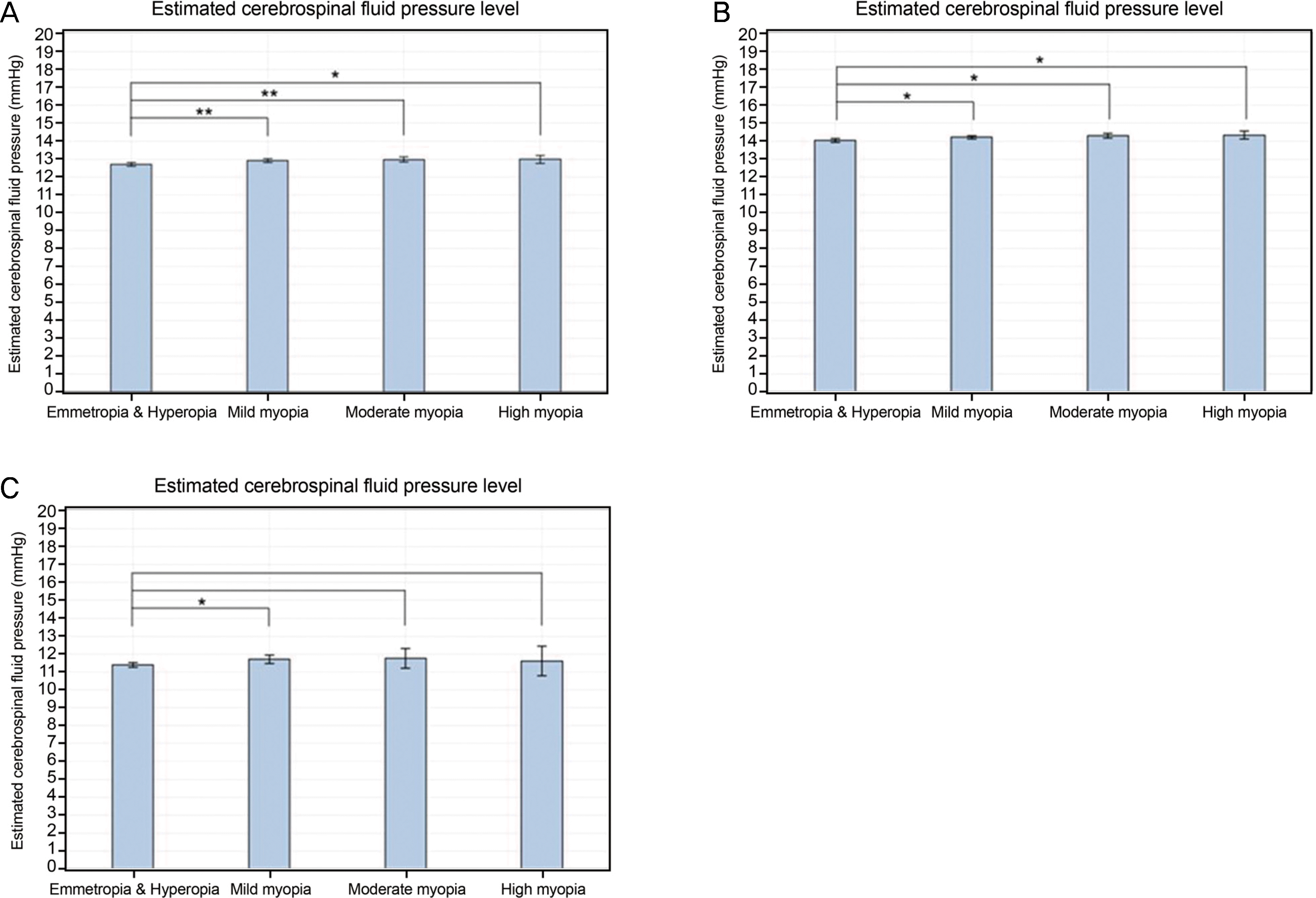 | Figure 4.Degree of myopia and estimated cerebrospinal fluid pressure. Estimated cerebrospinal fluid pressure according to the degree of myopia in the total study population (A), young (Group 1) (B), and old (C) age group (Group 2). *0.001 ≤ p < 0.05; ** p < 0.001. |
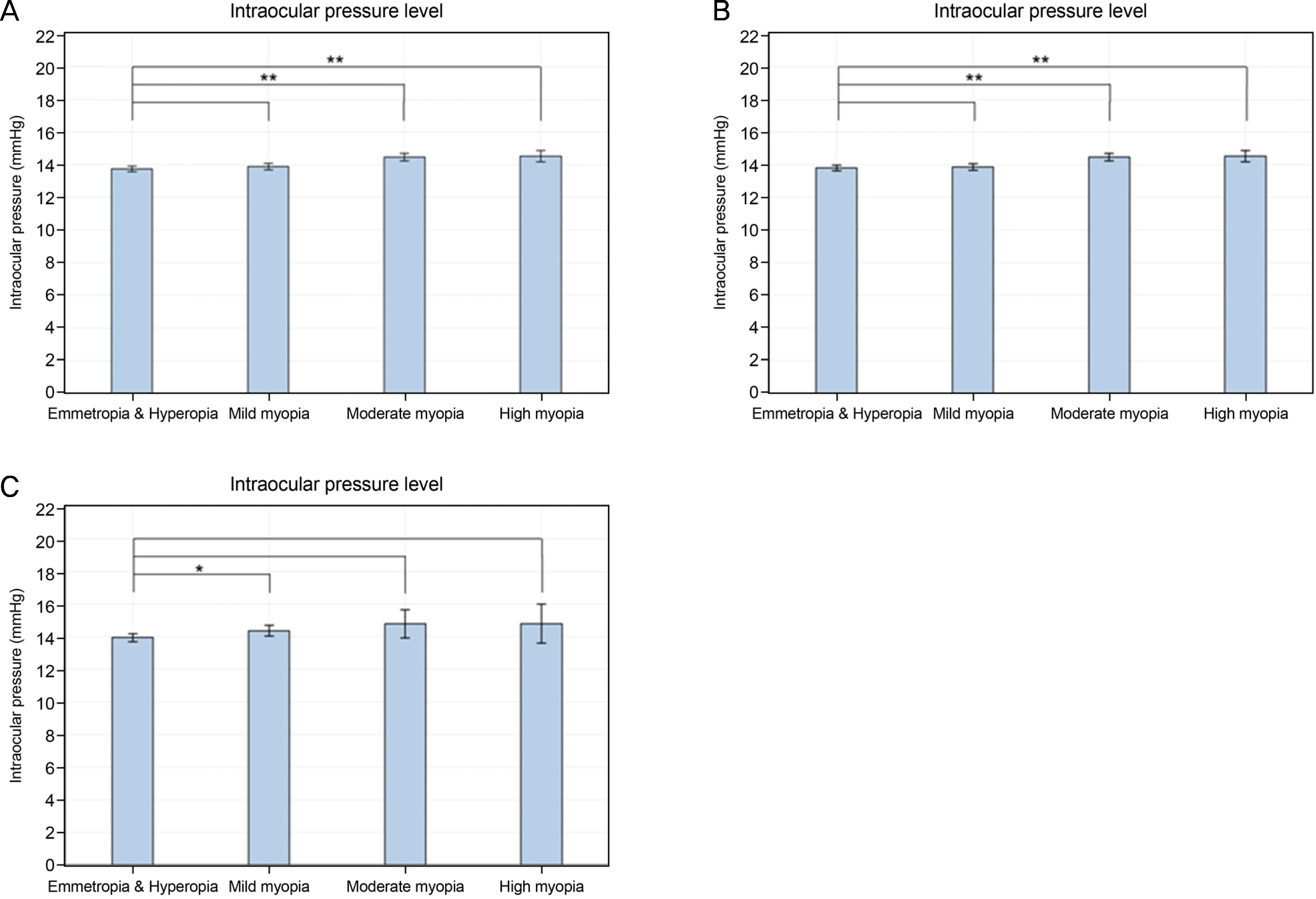 | Figure 5.Degree of myopia and intraocular pressure. Intraocular pressure according to the degree of myopia in the total study population (A), young (Group 1) (B), and old (C) age group (Group 2). *0.001 ≤ p < 0.05; ** p < 0.001. |
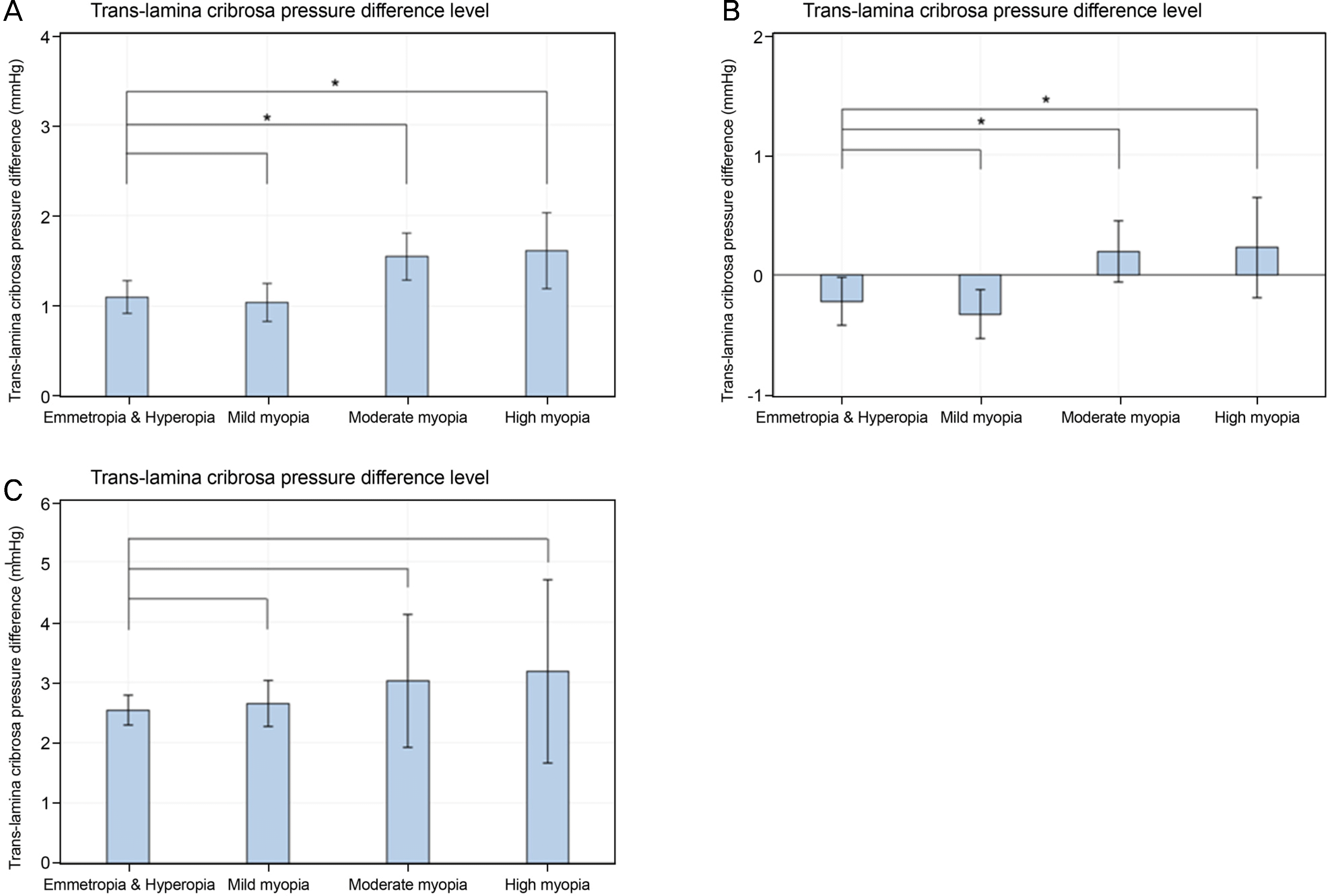 | Figure 6.Degree of myopia and trans-lamina pressure difference. Trans-lamina pressure difference according to the degree of myopia in the total study population (A), young (Group 1) (B), and old age group (Group 2) (C). *0.001 ≤ p < 0.05. |
Table 1.
Demographics and general health characteristics of the study population
| Parameter | Total study population (n = 6,740) | Young age group (19–49, n = 5,275) | Old age group (≥50, n = 1,465) | p-value† |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 38.0 ± 0.2 | 32.3 ± 0.2 | 56.1 ± 0.2 | <0.001 |
| Female (%) | 49.8 ± 0.6 | 48.6 ± 0.7 | 53.4 ± 1.4 | 0.001 |
| Area of residence (%) | 0.503 | |||
| Urban region | 73.7 ± 1.9 | 73.4 ± 2.0 | 74.9 ± 2.2 | |
| Rural region | 26.3 ± 1.9 | 26.6 ± 2.0 | 25.1 ± 2.2 | |
| Medical comorbidities (%) | ||||
| Hypertension | 17.9 ± 0.7 | 13.3 ± 0.6 | 40.3 ± 1.6 | <0.001 |
| Diabetes mellitus | 4.0 ± 0.3 | 2.4 ± 0.3 | 11.9 ± 1.1 | <0.001 |
| Hypercholesterolemia | 7.3 (0.4) | 5.5 (0.3) | 15.7 (1.1) | <0.001 |
| Anthropometric measurements | ||||
| Height (cm) | 165.5 ± 0.1 | 166.4 ± 0.1 | 161.4 ± 0.3 | <0.001 |
| Weight (kg) | 64.5 ± 0.2 | 64.7 ± 0.2 | 63.3 ± 0.3 | <0.001 |
| BMI (%) | <0.001 | |||
| <25 kg/m2 | 70.5 ± 0.7 | 72.0 ± 0.7 | 63.1 ± 1.4 | |
| ≥25 kg/m2 | 29.5 ± 0.7 | 28.0 ± 0.7 | 36.9 ± 1.4 | |
| Pressure parameters (mmHg) | ||||
| Systolic BP | 114.6 ± 0.2 | 111.7 ± 0.3 | 123.2 ± 0.6 | <0.001 |
| Diastolic BP | 76.4 ± 0.2 | 75.3 ± 0.3 | 80.3 ± 0.4 | <0.001 |
| Estimated CSFP | 13.7 ± 0.1 | 14.2 ± 0.1 | 11.5 ± 0.1 | <0.001 |
| IOP | 14.0 ± 0.1 | 14.0 ± 0.1 | 14.1 ± 0.1 | <0.001 |
| TLCPD | 0.7 ± 0.1 | 0.3 ± 0.1 | 3.0 ± 0.2 | <0.001 |
| Refractive error (diopter) | –1.3 (0.0) | –1.8 (0.0) | –0.3 (0.1) | <0.001 |
| Refractive status* (%) | <0.001 | |||
| Emmetropia & hyperopia | 37.1 ± 0.7 | 30.2 ± 0.6 | 70.3 ± 1.4 | |
| Mild myopia | 42.4 ± 0.6 | 46.2 ± 0.7 | 23.8 ± 1.3 | |
| Moderate myopia | 15.2 ± 0.5 | 17.4 ± 0.6 | 4.3 ± 0.6 | |
| High myopia | 5.3 ± 0.4 | 6.1 ± 0.4 | 1.6 ± 0.4 |
BMI = body mass index; BP = blood pressure; CSFP = cerebrospinal fluid pressure; IOP = intraocular pressure; TLCPD = trans-lamina cribrosa pressure difference.
Table 2.
Multivariate analysis of association between refractive error (D) and three pressure parameters (estimated cerebrospinal pressure, intraocular pressure, and trans-lamina cribrosa pressure difference) in total, young age, and old age population
| Parameters |
Model 1* |
Model 2† |
||||
|---|---|---|---|---|---|---|
| Beta | SE | p-value | Beta | SE | p-value | |
| Estimated cerebrospinal fluid pressure | ||||||
| Total population | –0.04 | 0.02 | 0.033‡ | –0.03 | 0.01 | <0.001‡ |
| Young age group (19–49) | –0.03 | 0.02 | 0.046‡ | –0.03 | 0.01 | <0.001‡ |
| Old age group (≥50) | –0.07 | 0.05 | 0.135 | –0.05 | 0.02 | 0.029‡ |
| Intraocular pressure | ||||||
| Total population | –0.12 | 0.02 | <0.001‡ | –0.11 | 0.02 | <0.001‡ |
| Young age group (19–49) | –0.12 | 0.02 | <0.001‡ | –0.11 | 0.02 | <0.001‡ |
| Old age group (≥50) | –0.12 | 0.05 | 0.010‡ | –0.09 | 0.05 | 0.065 |
| Trans-lamina cribrosa pressure difference | ||||||
| Total population | –0.07 | 0.02 | 0.001‡ | –0.08 | 0.02 | <0.001‡ |
| Young age group (19–49) | –0.07 | 0.03 | 0.002‡ | –0.08 | 0.02 | <0.001‡ |
| Old age group (≥50) | –0.05 | 0.06 | 0.468 | –0.04 | 0.05 | 0.287 |
Table 3.
Multivariate analysis of association between the degree of myopia and three pressure parameters (estimated cerebrospinal pressure, intraocular pressure, and trans-lamina cribrosa pressure difference) in total, young age, and old age population
| Parameters |
Model 1† |
Model 2‡ |
||||
|---|---|---|---|---|---|---|
| Beta | SE | p-value | Beta | SE | p-value | |
| Estimated cerebrospinal fluid pressure | ||||||
| Total population | 0.13 | 0.05 | 0.006* | 0.08 | 0.02 | <0.001* |
| Young age group (19–49) | 0.12 | 0.05 | 0.013* | 0.08 | 0.02 | <0.001* |
| Old age group (≥50) | 0.28 | 0.13 | 0.031* | 0.13 | 0.05 | 0.023* |
| Intraocular pressure | ||||||
| Total population | 0.30 | 0.05 | <0.001* | 0.29 | 0.04 | <0.001* |
| Young age group (19–49) | 0.29 | 0.05 | <0.001* | 0.28 | 0.05 | <0.001* |
| Old age group (≥50) | 0.42 | 0.14 | 0.003* | 0.36 | 0.14 | 0.010* |
| Trans-lamina cribrosa pressure difference | ||||||
| Total population | 0.15 | 0.06 | 0.006* | 0.18 | 0.05 | <0.001* |
| Young age group (19–49) | 0.17 | 0.07 | 0.009* | 0.17 | 0.06 | 0.003* |
| Old age group (≥50) | 0.13 | 0.19 | 0.483 | 0.25 | 0.16 | 0.080 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download