Abstract
Purpose
To investigate the occurrence and increase in intraocular pressure (IOP) over time in patients with retinal vein occlusion treated with dexamethasone intravitreal implants.
Methods
Thirty-two patients who met retreatment eligibility criteria received sequential intravitreal dexamethasone implant insertion for retinal vein occlusion in one eye, and were followed for 36 months (maximum of 10 injections) from February 2011 to March 2017. Patients without follow-up data at each investigation time point were excluded.
Results
An IOP increase ≥ 10 mmHg from baseline or an IOP ≥ 25 mmHg occurred in 18.8% of the patients. IOP-lowering medications were used in these patients. The first IOP elevation occurred with a mean of 5.9 months after the first intravitreal dexamethasone implant insertion in the IOP elevation group, and with a mean of 1.5 months after previous intravitreal dexamethasone implant insertion in each IOP elevation event. No patients required laser or incisional glaucoma surgery during the study period.
Conclusions
An increase in IOP occurred in the early period after intravitreal dexamethasone implant insertion, and did not occur in sequential insertion. Although an increase in IOP was a significant side effect of intravitreal dexamethasone implant insertion, the IOP could be controlled with careful observation and use of preliminary and therapeutic anti-ocular hypertensive agents.
Go to : 
References
1. Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010; 117:313–9.e1.

2. Sachdev A, Edington M, Morjaria R, Chong V. Comparing micro-perimetric and structural findings in patients with branch retinal vein occlusion and diabetic macular edema. Retina 2017 Nov 22. doi:. DOI: 10.1097/IAE.0000000000001961. [Epub ahead of print].

3. Basu S, Monira S, Modi RR, et al. Degree, duration, and causes of visual impairment in eyes affected with ocular tuberculosis. J Ophthalmic Inflamm Infect. 2014; 4:3.

4. Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1094–101.e5.

5. Finkelstein D. Argon laser photocoagulation for macular edema in branch vein occlusion. Ophthalmology. 1986; 93:975–7.

6. Yeh PC, Ramanathan S. Latanoprost and clinically significant abdominal macular edema after uneventful phacoemulsification with abdominal lens implantation. J Cataract Refract Surg. 2002; 28:1814–8.
7. Arcieri ES, Santana A, Rocha FN, et al. Blood-aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: a 6-month randomized trial. Arch Ophthalmol. 2005; 123:186–92.
8. Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014; 121:1904–14.

9. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone abdominal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118:2453–60.
11. Jiménez-Gómez B, González-Montpetit M, Fonollosa Calduch A, et al. Effects of ozurdex on intraocular pressure. A real life clinical practice study. Arch Soc Esp Oftalmol. 2015; 90:421–5.

12. Maturi RK, Pollack A, Uy HS, et al. Intraocular pressure in patients with diabetic macular edema treated with dexamethasone abdominal implant in the 3-year MEAD Study. Retina. 2016; 36:1143–52.
13. Singer MA, Capone A Jr, Dugel PU, et al. Two or more abdominal intravitreal implants as monotherapy or in combination therapy for macular edema in retinal vein occlusion: subgroup analysis of a retrospective chart review study. BMC Ophthalmol. 2015; 15:33.

14. Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal abdominal for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011; 31:915–23.
15. Meyer LM, Schönfeld CL. Secondary glaucoma after intravitreal dexamethasone 0.7 mg implant in patients with retinal vein abdominal: a one-year follow-up. J Ocul Pharmacol Ther. 2013; 29:560–5.
16. Lee MW, Kyung SE, Chang MH. Prophylactic effect of abdominal 0.15% on IOP elevation after intravitreal triamcinolone abdominal injection. J Korean Ophthalmol Soc. 2008; 49:743–52.
17. Han JB, Seo KH, Yu SY. The effect of prophylactic IOP-lowering medication after intravitreal dexamethasone implantation. J Korean Ophthalmol Soc. 2014; 55:1828–33.

18. Zalewski D, Raczyń ska D, Raczyń ska K. Five-month observation of persistent diabetic macular edema after intravitreal injection of Ozurdex implant. Mediators Inflamm. 2014; 2014:364143.

19. Guigou S, Hajjar C, Parrat E, et al. Multicenter Ozurdex(R) abdominal for diabetic macular edema: MOZART study. J Fr Ophtalmol. 2014; 37:480–5.
20. Saatci AO, Doruk HC, Yaman A. Intravitreal dexamethasone implant (ozurdex) in coats' disease. Case Rep Ophthalmol. 2013; 4:122–8.

21. Kapoor KG, Wagner MG, Wagner AL. The sustained-release abdominal implant: expanding indications in vitreoretinal disease. Semin Ophthalmol. 2015; 30:475–81.
22. Park DH, Ha SJ, Lee SJ. Intraocular pressure elevation after 0.7 mg intravitreal dexamethasone (Ozurdex(R)) implantation: a one year follow-up. J Korean Ophthalmol Soc. 2015; 56:891–9.
23. Kubota T, Okabe H, Hisatomi T, et al. Ultrastructure of the abdominal meshwork in secondary glaucoma eyes after intravitreal triamcinolone acetonide. J Glaucoma. 2006; 15:117–9.
24. Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006; 17:163–7.
25. Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a abdominal of the literature. Eye (Lond). 2006; 20:407–16.
26. Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999; 18:629–67.

27. Lowder C, Belfort R Jr, Lightman S, et al. Dexamethasone abdominal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011; 129:545–53.
Go to : 
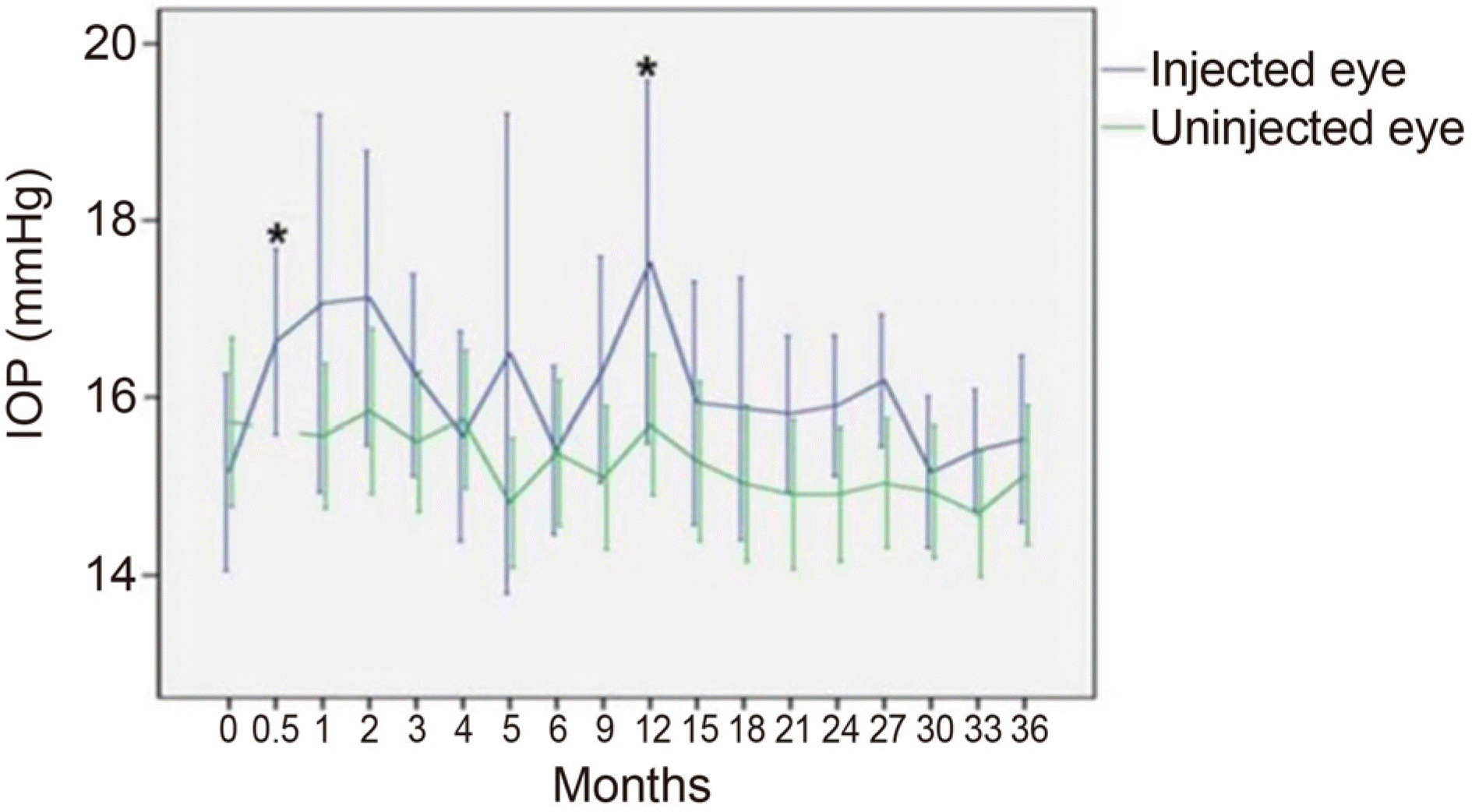 | Figure 1.Mean intraocular pressure (IOP) changes in eyes treated with dexamethasone intravitreal implant and in the fellow eyes during the 36-month follow up. Bar is 95% confidence interval for the mean. *Statistical significant difference of mean IOP compared to the fellow eye (p ≤ 0.05, Mann-Whitney U-test). |
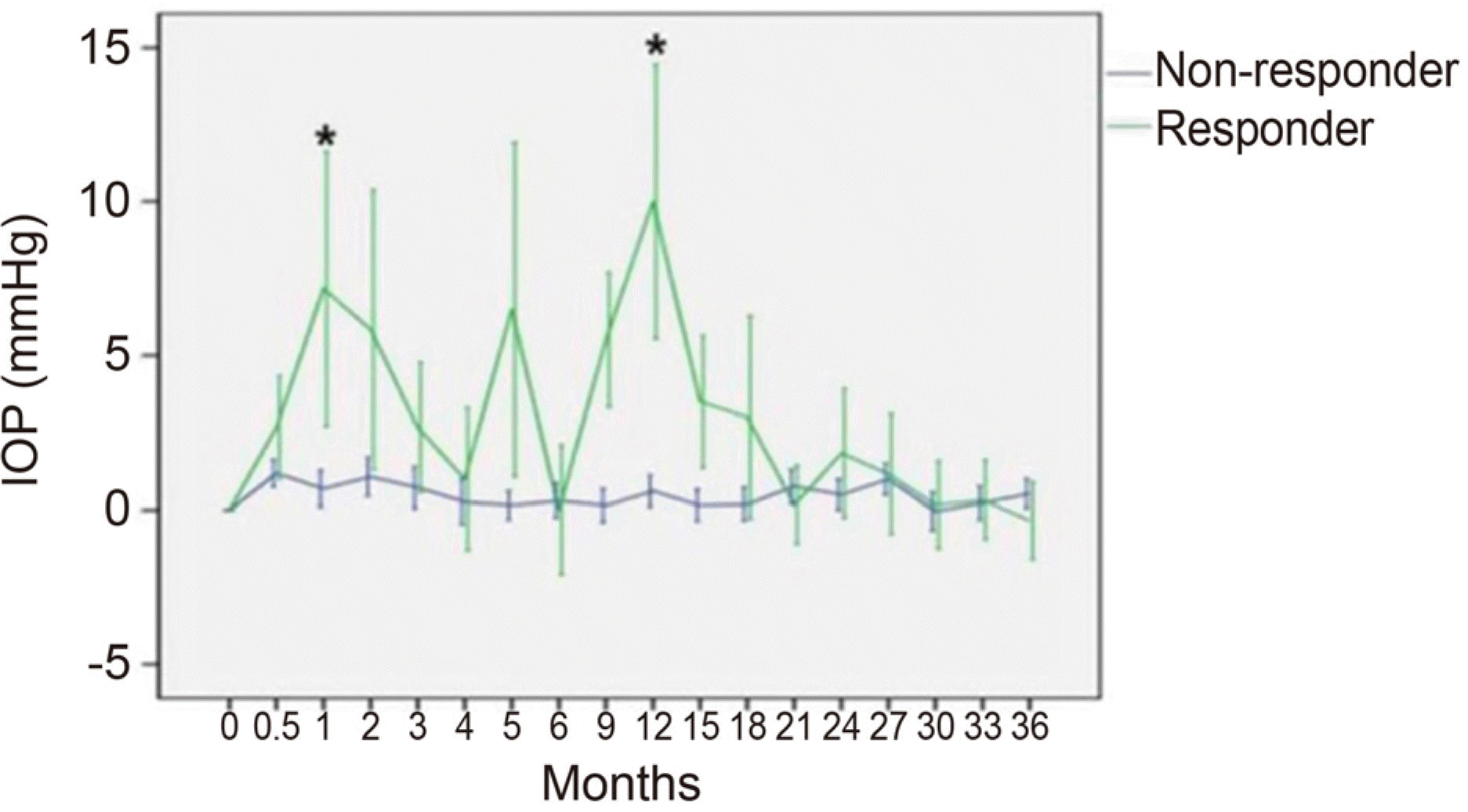 | Figure 2.Mean increased intraocular pressure (IOP) changes in eyes treated with dexamethasone intravitreal implant from baseline IOP of the responder (n = 6) and non-responder group (n = 26) during 36 months follow up. Bar is 95% confidence interval for mean. * Statistical significant difference of mean IOP compared to the fellow eye (p ≤ 0.05, Mann-Whitney U-test). |
 | Figure 3.Intraocular pressure (IOP) changes in the responder patients. (A) IOP change in the responder patient A. (B) IOP change in the responder patient B. (C) IOP change in the responder patient C. (D) IOP change in the responder patient D. (E) IOP change in the responder patient E. (F) IOP change in the responder patient F. Bar: span of time of anti-ocular hypertensive eyedrop use. * Intravitreal dexamethasone implant insertion; ¥ Mannitol intravenous injection. |
Table 1.
Demographic data
Table 2.
Classification and demographics of responder and non-responder group
| Parameters | Steroid responder group (n = 6) | Steroid non-responder group (n = 26) | p-value |
|---|---|---|---|
| Female gender (n, %) | 2 (33.3) | 19 (73.1) | 0.148† |
| Age (years) | 64.5 ± 10.5 | 60.0 ± 12.6 | 0.439* |
| Right eye (n, %) | 4 (66.7) | 13 (50) | 0.196† |
| Pseudophakic eyes (n, %) | 5 (83.3) | 9 (34.6) | 0.637† |
| Vitrectomized eyes (n, %) | 0 (0) | 2 (7.7) | 0.661† |
| Diabetes (n, %) | 1 (16.7) | 0 (0) | 1.000† |
| Hypertension (n, %) | 0 (0) | 0 (0) | 1.000† |
| Hypertension and DM (n, %) | 1 (16.7) | 6 (23.1) | 0.625† |
| Duration of symptom (months) | 15.6 ± 9.0 | 12.2 ± 10.1 | 0.387* |
| Number of treatments | 5.2 ± 2.5 | 6.0 ± 2.9 | 0.608* |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download