Abstract
Purpose
To compare the short-term efficacy of oral spironolactone vs. observation in patients with acute central serous chorioretinopathy.
Methods
Forty-seven eyes of 47 patients diagnosed as acute central serous chorioretinopathy from January 2013 to June 2016 were treated with oral spironolactone or were observed. This was a retrospective study involving patients analyzed for changes in best-corrected visual acuity (BCVA), central macular thickness (CMT), and subretinal fluid height (SRFH).
Results
Oral spironolactone was used to treat 24 eyes and 23 eyes were observed. There were no differences in baseline characteristics including age, sex, BCVA (logMAR), CMT, and SRFH between the two groups. The mean BCVA, CMT, and SRFH improved compared with baseline at 1 month and 2 months in both groups. In comparison between the two groups, the mean BCVA of oral spironolactone group improved more than in the observation group at 2 months (p = 0.006). There was a significant difference in CMT between the two groups at 1 month and 2 months (p = 0.017 and p < 0.001, respectively), and there was a significant difference in subretinal fluid height between the two groups at 2 months (p = 0.007). Complete resolution of subretinal fluid was achieved in 33.3% (8/24) and 21.7% (5/23) of the eyes in the oral spironolactone group and the observation group, respectively, at 2 months (p = 0.374). There was no serious side effect in patients treated with oral spironolactone.
Conclusions
Both oral spironolactone and observation were effective for the treatment of acute central serous chorioretinopathy. Oral spironolactone was more effective than observation when comparing the best-corrected visual acuity, central macular thickness, and subretinal fluid height. As a noninvasive method for treatment of acute central serous chorioretinopathy, oral spironolactone showed anatomical improvement and improved visual acuity during a short-term period.
Go to : 
References
1. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967; 63(Suppl):1–139.
2. Daruich A, Matet A, Marchionno L, et al. Acute central serous abdominal: factors influencing episode duration. Retina. 2017; 37:1905–15.
3. Jalkh AE, Jabbour N, Avila MP, et al. Retinal pigment epithelium decompensation. I. Clinical features and natural course. Ophthalmology. 1984; 91:1544–8.
4. Ie D, Yannuzzi LA, Spaide RF, et al. Subretinal exudative deposits in central serous chorioretinopathy. Br J Ophthalmol. 1993; 77:349–53.

5. Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular abdominal in chronic central serous chorioretinopathy. Retina. 2003; 23:1–7. quiz 137–8.
6. Ficker L, Vafidis G, While A, Leaver P. abdominal follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988; 72:829–34.
7. Yeo YD, Kim JH, Kim YC, Kim KS. Photodynamic therapy and focal laser photocoagulation in chronic central serous choriretinopathy. J Korean Opthalmol Soc. 2016; 57:56–62.
8. Kim YS, Lee YH, Kim HS, et al. Comparison of therapeutic effect between half-energy photodynamic therapy and intravitreal abdominal injection in chronic central serous chorioretinopathy for 12 months. J Korean Opthalmol Soc. 2013; 54:1526–33.
9. Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013; 58:103–26.

10. Mirshahi M, Nicolas C, Mirshahi A, et al. The mineralocorticoid hormone receptor and action in the eye. Biochem Biophys Res Commun. 1996; 219:150–6.

11. Zhao M, Célérier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012; 122:2672–9.

12. Ghadiali Q, Jung JJ, Yu S, et al. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a one-year pilot study. Retina. 2016; 36:611–8.
13. Bousquet E, Beydoun T, Rothschild PR, et al. Spironolactone for nonresolving central serous chorioretinopathy: a randomized abdominalled crossover study. Retina. 2015; 35:2505–15.
14. Daruich A, Matet A, Dirani A, et al. Oral mineralocorticoid-receptor antagonists: real-life experience in clinical subtypes of nonresolving central serous chorioretinopathy with chronic epitheliopathy. Transl Vis Sci Technol. 2016; 5:2. eCollection 2016 Mar.

15. Aronova A, Fahey TJ III, Zarnegar R. Management of abdominal in primary aldosteronism. World J Cardiol. 2014; 6:227–33.
16. Desai AS, Lewis EF, Li R, et al. Rationale and design of the abdominal of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of abdominal in patients with symptomatic heart failure and abdominal ejection fraction. Am Heart J. 2011; 162:966–72.e10.
17. de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of abdominal therapy in patients with true resistant hypertension. Hypertension. 2010; 55:147–52.
18. Herold TR, Rist K, Priglinger SG, et al. abdominal results and abdominal rates after spironolactone treatment in nonresolving abdominal serous abdominal (CSCR). Graefes Arch Clin Exp Ophthalmol. 2017; 255:221–9.
19. Falavarjani KG, Amirsardari A, Habibi A, et al. Visual and abdominal outcomes of spironolactone therapy in patients with chronic abdominal serous chorioretinopathy. J Ophthalmic Vis Res. 2017; 12:281–9.
20. Comez-Sanchez E, Gomez-Sanchez CE. The multifaceted abdominal receptor. Compr Physiol. 2014; 4:965–94.
21. Bousquet E, Beydoun T, Zhao M, et al. Mineralocorticoid receptor antagonism in the treatment of chronic central serous abdominal: a pilot study. Retina. 2013; 33:2096–102.
22. Sun X, Shuai Y, Fang W, et al. Spironolactone versus observation in the treatment of acute central serous chorioretinopathy. Br J Ophthalmol 2017 Oct 31. pii: bjophthalmol-2017–311096.doi:. DOI: 10.1136/bjophthalmol-2017-311096. [Epub ahead of print].

Go to : 
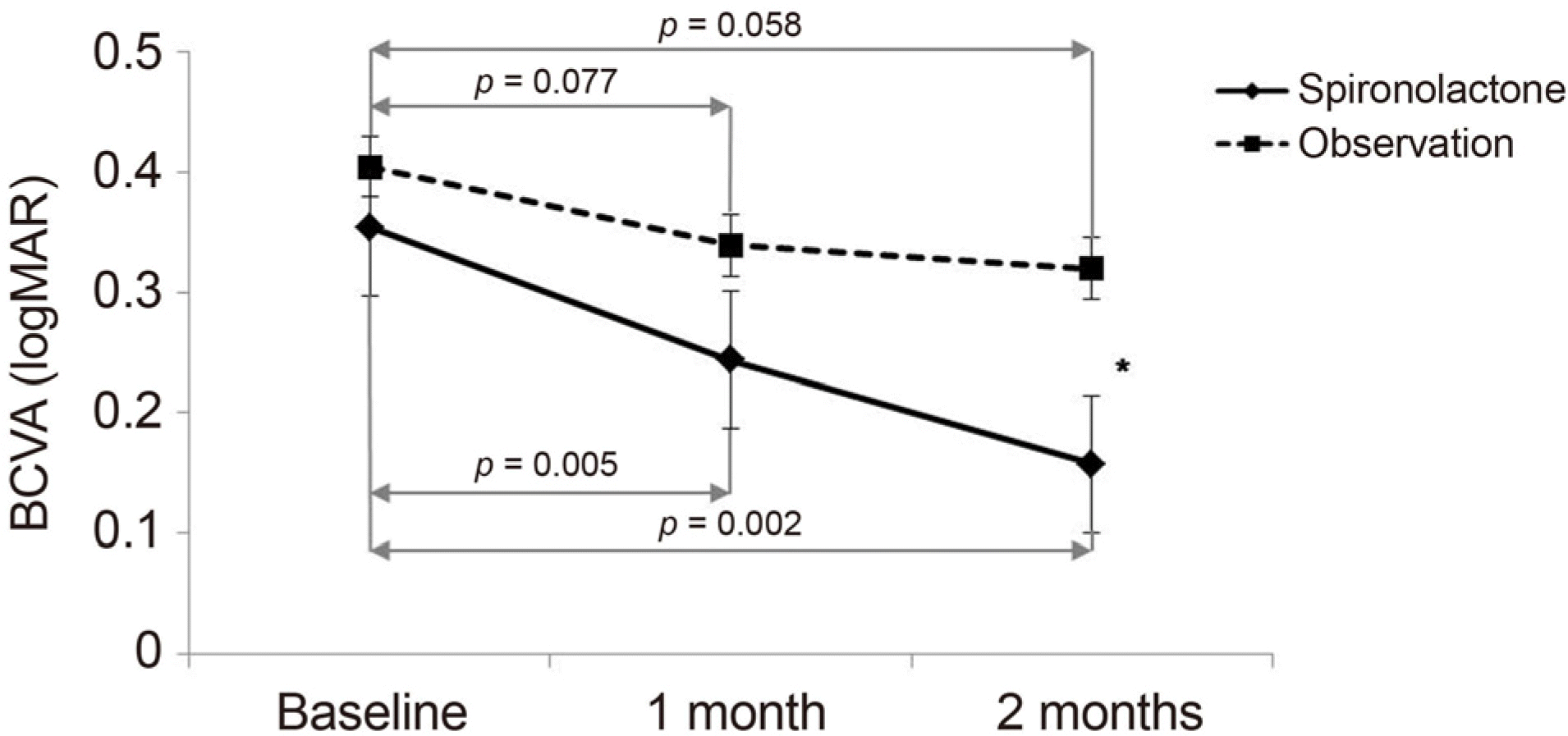 | Figure 1.Comparison of mean best corrected visual acuity (BCVA, logMAR) between the groups over time. Spironolactone group showed significant difference at 1 and 2 months compared with baseline. No significant difference was found during follow-up period in observation group. There was a statistically significant difference between spironolactone and observation group at 2 months. Error bars represent standard errors. * p < 0.05 compared between groups. |
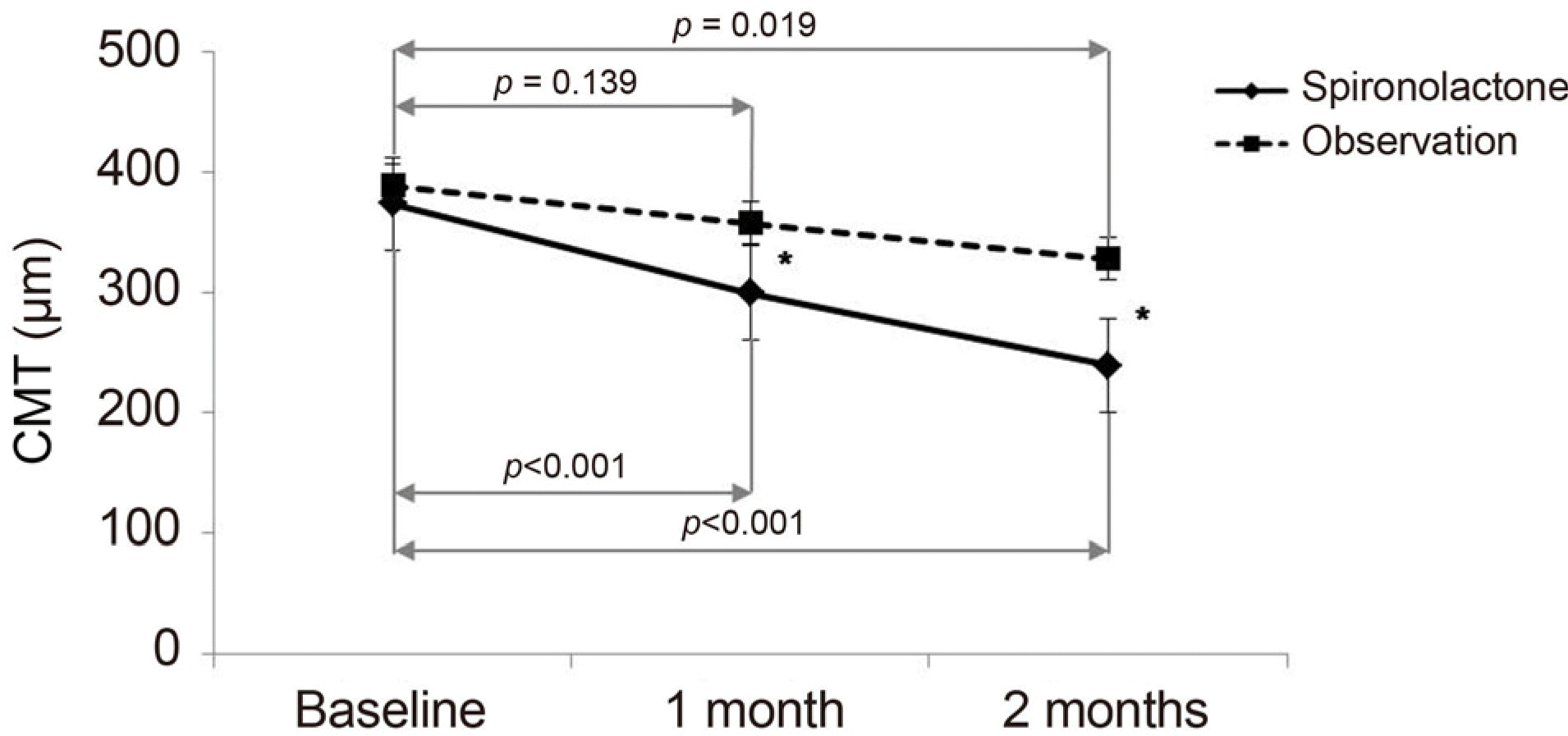 | Figure 2.Comparison of mean central macular thickness (CMT, μ m) between the groups over time. Spironolactone group showed significant difference at 1 and 2 months compared with baseline. In observation group, a significant decrease was shown at 2 months. A significant difference in CMT between the groups was observed at 1 and 2 months. Error bars *p < 0.05 compared between represent standard errors. groups. |
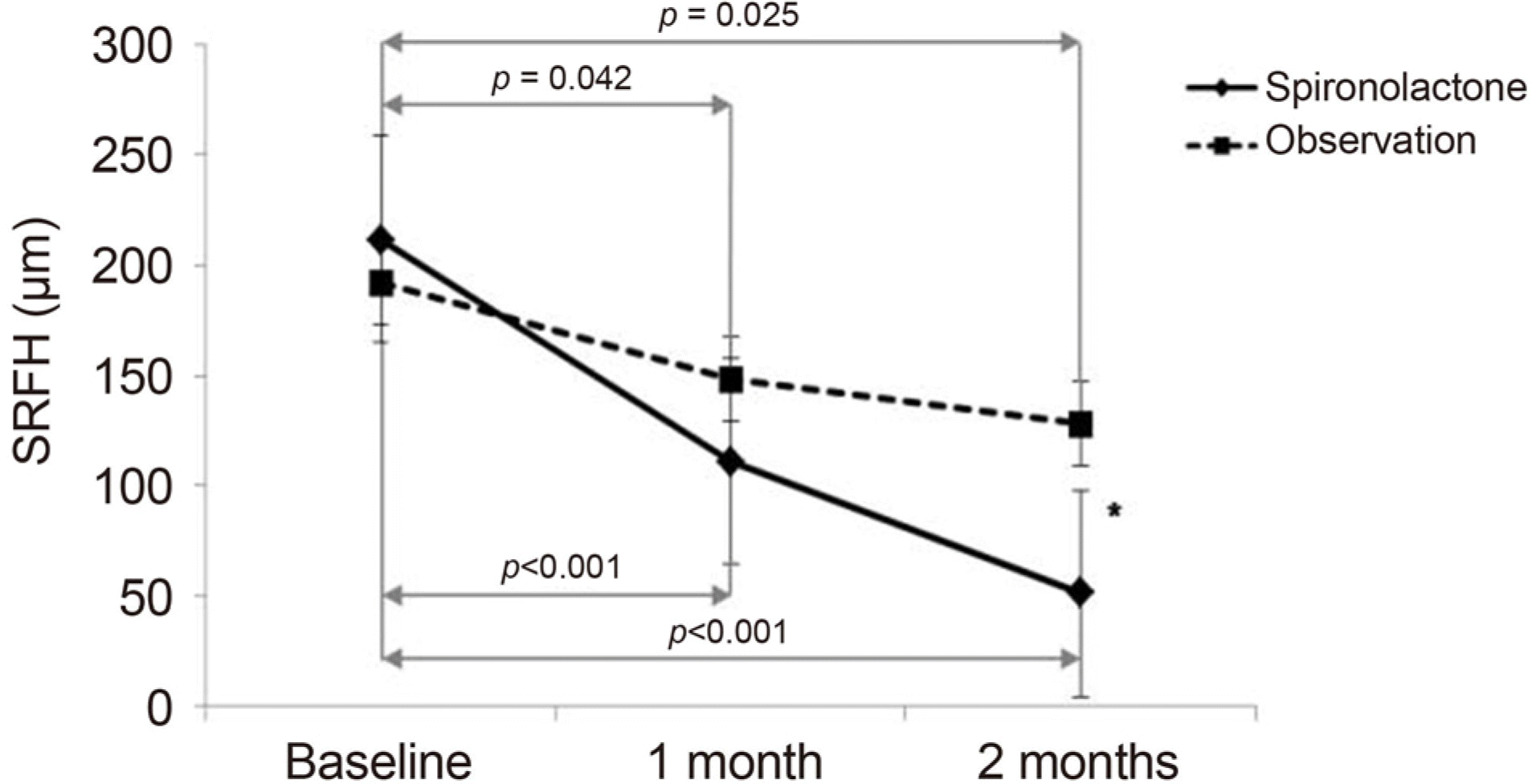 | Figure 3.Comparison of mean subretinal fluid height (SRFH, μ m) between the groups over time. Both groups showed a significant difference at 1 month, 2 months compared with baseline. A significant difference in SRFH between the groups was observed at 2 months. Error bars represent standard errors. * p < 0.05 compared between groups. |
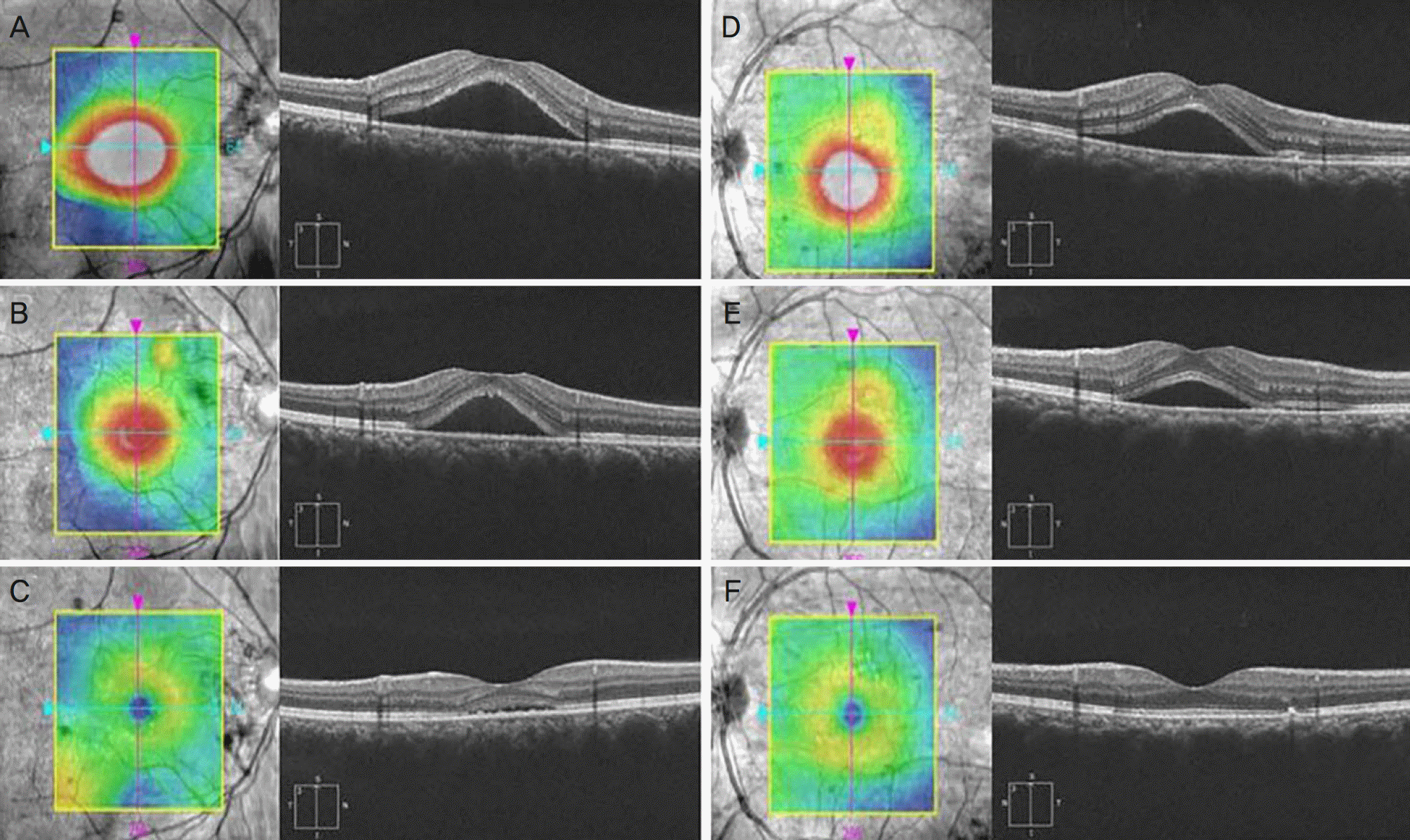 | Figure 4.Macular thickness map and optical coherence tomography (OCT) scan of the patients with central serous chorioretinopathy (CSC). OCT images of a 48-year-old man with CSC at baseline (A), at 1 month (B), and 2 months (C) after observation. OCT images of 54-years-old man with CSC at baseline (D), at 1 month (E), and 2 months (F) after oral spironolactone. |
Table 1.
Baseline characteristics of patients in the two groups
| Spironolactone (n = 24) | Control (n = 23) | p-value | |
|---|---|---|---|
| Age (years) | 47.54 ± 8.29 | 44.90 ± 8.26 | 0.277* |
| Sex (male:female) | 19:5 | 16:7 | 0.793† |
| Diabetes (n, %) | 1 (4.2) | 2 (8.5) | 0.472‡ |
| Hypertension (n, %) | 7 (29.2) | 5 (21.8) | 0.641† |
| Symptom duration (weeks) | 7.11 ± 1.35 | 6.82 ± 0.80 | 0.352* |
| Follow up period (weeks) | 10.61 ± 2.58 | 10.64 ± 2.22 | 0.876* |
Table 2.
Changes in BCVA, CMT and SRFH during the follow-up period in the two groups
|
Spironolactone (n = 24) |
Control (n = 23) |
p-value† | |||
|---|---|---|---|---|---|
| Compared to the baseline | p-value* | Compared to the baseline | p-value* | ||
| BCVA (logMAR) | |||||
| Baseline | 0.35 ± 0.25 | 0.40 ± 0.25 | 0.492 | ||
| 1 month | 0.24 ± 0.18 | 0.005 | 0.34 ± 0.21 | 0.077 | 0.105 |
| 2 months | 0.16 ± 0.17 | 0.002 | 0.32 ± 0.19 | 0.058 | 0.006 |
| CMT (μ m) | |||||
| Baseline | 373.54 ± 96.97 | 388.48 ± 89.58 | 0.586 | ||
| 1 month | 299.18 ± 78.37 | <0.001 | 357.61 ± 80.01 | 0.139 | 0.017 |
| 2 months | 239.00 ± 41.74 | <0.001 | 327.35 ± 89.10 | 0.019 | <0.001 |
| SRFH (μ m) | |||||
| Baseline | 211.45 ± 92.86 | 192.04 ± 72.94 | 0.431 | ||
| 1 month | 111.00 ± 88.87 | <0.001 | 148.22 ± 104.42 | 0.042 | 0.219 |
| 2 months | 51.28 ± 53.89 | <0.001 | 128.04 ± 112.72 | 0.025 | 0.007 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download