Abstract
Purpose
To investigate the possibility of transplantation into rabbits of collagenase-induced cultured human corneal endothelial cell plate-rabbit corneal stromal complexes.
Methods
Human corneal endothelial cells were isolated from residual corneal limbus samples and treated with collagenase to create corneal endothelial cell sheets. Cultured sheets were transplanted into the rabbit stroma after the rabbit corneal endothelial cells and Descemet's membrane were removed. Hematoxylin-and-eosin staining and immunofluorescence staining for collagen VIIIa2 (COL8A2) and zonula occludens-1 (ZO-1) were performed. The cultured human corneal endothelial cell sheet-rabbit corneal stromal complex was transplanted into rabbits. On days 3, 5, and 7, the transplanted corneas were photographed and corneal opacity was measured. One week later, the rabbits were sacrificed. Hematoxylin-and-eosin staining and immunofluorescence staining for COL8A2 were performed.
Results
The cultured cells were immunofluorescently stained for collagen VIIIa1 and ZO-1. Collagenase-treated cultured human corneal endothelial cells formed monolayers on day 7 after transplantation into the rabbit corneal stroma and immunofluorescently stained for COL8A2 and ZO-1. The cultured human corneal endothelial cell sheet-rabbit stroma complex transplanted into rabbits was transparent on days 3 and 5, but corneal opacity developed by day 7. Histologic examination revealed 3,3′-dioctadecyloxacarbocyanine perchlorate (DIO)-stained corneal endothelium (green) and hard-tissue lymphocytes had infiltrated the cultured corneal endothelial cell plate-rabbit corneal stromal complex graft group.
Figures and Tables
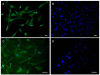 | Figure 1Human corneal endothelial cell (HCEC) culture and immunofluorescence staining. Cultured HCECs expressed collagen VIIIa2 (green) which is a marker of corneal endothelial cells (A), and ZO-1 (green) which is an indicator of tight junction (C). Negative staining was performed with the omission of a primary antibody (B, D). Scale bar = 50 µm. |
 | Figure 2Detachment and reattachment of cultured human corneal endothelial cell sheets. (A) Cultured human corneal endothelial cell sheets using collagenase were detached from the bottom of the cell dishes and floating in the media (black arrow). (B) Cultured human corneal endothelial cell sheets using collagenase were reattached onto the fibronectin and collagen-coated slides (black arrow). (C) Cultured human corneal endothelial cell sheets using collagenase were immune-stained with ZO-1 (green) after reattachments. |
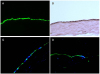 | Figure 3Histology of 3,3′-dioctadecyloxacarbocyanine perchlorate (DIO), hematoxylin and eosin staining and immunofluorescent staining of COL8A2 and ZO-1 of cultured human corneal endothelial cell sheets-rabbit corneal stroma complex. (A) DIO-stained cells (green) were identified on the corneal stroma. (B) Hematoxylin and eosin staining showed the mono-layer human corneal endothelial cells on the corneal stroma. (C, D) Transplanted human corneal endothelial cell sheets expressed COL8A2 (C; green) and ZO-1 (D; green) on the corneal stroma. Magnification ×200. |
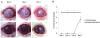 | Figure 4Representative pictures of in vivo transplantation of cultured human corneal endothelial cell sheets-rabbit corneal stroma complex (A) and corneal opacity grade (B). (A) Cultured human corneal endothelial cell sheets-rabbit corneal stroma complex (3 photos above) showed more transparent compared with control (3 photos below). (B) Corneal opacity grade in the experimental group was lower compared to the control group at day 1, 3 and 5. At day 7, cornea in the experimental group became more opaque than baseline. *Statistiscally significant by independent t-test. |
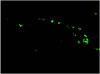 | Figure 5Histology showed 3,3′-dioctadecyloxacarbocyanine perchlorate (DIO)-stained cells (green) on the corneal endothelium at day 7. Magnification ×200. |
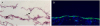 | Figure 6Hematoxylin and eosin staining (A), and immune fluorescence staining with collagen type VIII alpha 2 (B) at day 7. (A) Transplanted human corneal endothelial cells were on the inner surface of cornea. A few inflammatory cells were shown (black arrow), which indicates the graft rejection or graft primary failure. (B) Collagen type VIII alpha 2-expressing cells were on the inner surface of cornea. Magnification ×200. |
References
1. Geroski DH, Edelhauser HF. Quantitation of Na/K ATPase pump sites in the rabbit corneal endothelium. Invest Ophthalmol Vis Sci. 1984; 25:1056–1060.
2. Dikstein S. Efficiency and survival of the corneal endothelial pump. Exp Eye Res. 1973; 15:639–644.

3. Bourne WM, Johnson DH, Campbell RJ. The ultrastructure of Descemet's membrane. III. Fuchs' dystrophy. Arch Ophthalmol. 1982; 100:1952–1955.
4. Polack FM, Sugar A. The phacoemulsification procedure. III. Corneal complications. Invest Ophthalmol Vis Sci. 1977; 16:39–46.
5. Naumann GO. Corneal endothelial decompensation following intraocular surgery. Ophthalmology. 1985; 92:714.
6. Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Invest Ophthalmol Vis Sci. 2002; 43:2152–2159.
7. Bertelmann E, Pleyer U, Rieck P. Risk factors for endothelial cell loss post-keratoplasty. Acta Ophthalmol Scand. 2006; 84:766–770.

8. Olson RJ, Pingree M, Ridges R, et al. Penetrating keratoplasty for keratoconus: a long-term review of results and complications. J Cataract Refract Surg. 2000; 26:987–991.

9. Melles GR, Ong TS, Ververs B, van der Wees J. Preliminary clinical results of Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2008; 145:222–227.

10. Leyland M. Syringe or pump for air-assisted DSAEK. J Cataract Refract Surg. 2009; 35:2. author reply 2.

11. Terry MA, Shamie N, Chen ES, et al. Precut tissue for Descemet's stripping automated endothelial keratoplasty: vision, astigmatism, and endothelial survival. Ophthalmology. 2009; 116:248–256.
12. Park CY, Zhu Z, Zhang C, et al. Cellular redox state predicts in vitro corneal endothelial cell proliferation capacity. Exp Eye Res. 2006; 83:903–910.

13. Li W, Sabater AL, Chen YT, et al. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007; 48:614–620.

14. Mimura T, Yamagami S, Usui T, et al. Necessary prone position time for human corneal endothelial precursor transplantation in a rabbit endothelial deficiency model. Curr Eye Res. 2007; 32:617–623.

15. Lai JY, Chen KH, Hsiue GH. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation. 2007; 84:1222–1232.

16. Patel SV, Bachman LA, Hann CR, et al. Human corneal endothelial cell transplantation in a human ex vivo model. Invest Ophthalmol Vis Sci. 2009; 50:2123–2131.

17. Ple-Plakon PA, Shtein RM. Trends in corneal transplantation: indications and techniques. Curr Opin Ophthalmol. 2014; 25:300–305.
18. Li S, Liu L, Wang W, et al. Efficacy and safety of Descemet's membrane endothelial keratoplasty versus Descemet's stripping endothelial keratoplasty: a systematic review and meta-analysis. PLoS One. 2017; 12:e0182275.
19. Jhanji V, Mehta JS, Sharma N, et al. Targeted corneal transplantation. Curr Opin Ophthalmol. 2012; 23:324–329.

20. Yoshida J, Yokoo S, Oshikata-Miyazaki A, et al. Transplantation of human corneal endothelial cells cultured on bio-engineered collagen vitrigel in a rabbit model of corneal endothelial dysfunction. Curr Eye Res. 2017; 42:1420–1425.

21. Kobayashi A, Fujiki K, Murakami A, et al. Analysis of COL8A2 gene mutation in Japanese patients with Fuchs' endothelial dystrophy and posterior polymorphous dystrophy. Jpn J Ophthalmol. 2004; 48:195–198.

22. Kuot A, Mills R, Craig JE, et al. Screening of the COL8A2 gene in an Australian family with early-onset Fuchs' endothelial corneal dystrophy. Clin Exp Ophthalmol. 2014; 42:198–200.
23. Chen J, Li Z, Zhang L, et al. Descemet's membrane supports corneal endothelial cell regeneration in rabbits. Sci Rep. 2017; 7:6983.

24. Chen KH, Hsu WM, Lee SM. Differential effects of transforming growth factor-beta2 on corneal endothelial cell proliferation-A role of serum factors. Exp Eye Res. 2002; 75:61–67.
25. Koizumi N, Sakamoto Y, Okumura N, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007; 48:4519–4526.

26. Mimura T, Yamagami S, Yokoo S, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004; 45:2992–2997.

27. Van den Bogerd B, Ní Dhubhghaill S, Zakaria N. Characterizing human decellularized crystalline lens capsules as a scaffold for corneal endothelial tissue engineering. J Tissue Eng Regen Med. 2018; 12:e2020–e2028.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download