Dear Editor:
Bowen's disease (BD) is a squamous cell carcinoma in situ of the skin. Histologically, it presents as full-thickness dysplasia of the epidermis with a loss of normal maturation. Surgical excision might be the most effective treatment as it is associated with a low recurrence rate. However, when surgery is not an option, nonsurgical therapies can also be viable alternatives. Ingenol mebutate is a novel topical agent made from the plant Euphorbia peplus, and has been approved for a field-directed treatment of actinic keratosis (AK). Herein, we report a BD patient with a good treatment response to ingenol mebutate.
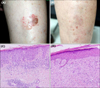
A 68-year-old woman who presented with a 3×4 cm sized brown-to-red plaque on her left shin came to our clinic. There was a bulla beside the lesion which was formed caused by burn after repetitive hot-massages on the lesion. A histopathologic examination was consistent with BD (Fig. 1A, C). The patient was informed the treatment options such as surgery and some alternatives such as radiation or topical treatment. After informed consent, she was to be treated with topical ingenol mebutate gel. The patient underwent a pretreatment of CO2 fractional laser to promote transcutaneous drug delivery (Mosaic eCO2 laser; Lutronic Corporation, Goyang, Korea) with fluence 100 mJ, power 30W, density 200 spots/cm2. She then was instructed to apply 0.05% ingenol mebutate gel for two consecutive days. Four weeks following the treatment, erythematous plaque had disappeared, leaving behind a slight hyperpigmentation. A follow-up skin biopsy was performed at 3 months post-treatment, and the result presented only irregular acanthosis with dermal fibrosis (Fig. 1D). Continued observation with thorough clinical inspections throughout the following 2 years revealed no signs of recurrence (Fig. 1B).
In our case, a surgical approach was not feasible because the size of the lesions was relatively large, posing a high risk of substantial scarring. We suggest that topical treatment using ingenol mebutate may be an effective and safe treatment alternative to surgical excision.
Ingenol mebutate, back in 2012, has been proven to be effective and safe as a field therapy for AK1. Since then, there have been many clinical trials with successful results and no significant adverse effects. The exact mechanism of action of ingenol mebutate is not fully understood thus far, but the dual mechanism theory has been generally accepted. Rosen et al.2 hypothesized that the mechanism of ingenol mebutate is both rapid lesion necrosis and specific neutrophil-mediated, antibody-dependent cellular cytotoxicity (ADCC). In a previous animal study, ingenol mebutate was shown to up-regulate the serum level of tumor-specific IgG and promote ADCC amongst residual cancer cells.
Recently, there have been some case reports that used ingenol mebutate gel to treat BD patients34. However, in our case, a fractional CO2 laser was performed prior to the application of ingenol mebutate gel. This was done because a study has reported that a fractional CO2 laser can enhance the absorption of topical agents5. Likewise, and as evidenced by our case, a pretreatment of factional CO2 laser may increase the efficacy of topical ingenol mebutate in BD patients. Moreover, the patient showed no recurrence in 2 years of follow-up period, and there have been no report of longer period of follow-up in the literature for our knowledge yet.
Herein, we report a case of BD successfully treated with ingenol mebutate gel and fractional ablation laser pretreatment. She showed an excellent treatment response with no recurrence for 2 years. Further investigations with a large number of cases may be necessary to clarify the efficacy and safety of topical ingenol mebutate gel in BD patients.
Figures and Tables
Fig. 1
(A) Bowen's disease lesion on the leg of the first case. (B) 2 years after application of 0.05% ingenol mebutate gel on the lesion combined with fractional carbondioxide laser. (C) Histopathologic image before treatment. There is full-thickness intraepidermal cellular atypia along with nuclear hyperchromasia and dyskeratotic cells (D) Follow-up skin biopsy revealed no evidance of recurrance observed. Only mild irregular acanthosis with dermal fibrosis presented (H&E; C, D: ×200).
References
1. Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012; 366:1010–1019.

2. Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J Am Acad Dermatol. 2012; 66:486–493.

3. Salleras Redonnet M, Quintana Codina M. Ingenol mebutate gel for the treatment of Bowen's disease: a case report of three patients. Dermatol Ther. 2016; 29:236–239.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download