Dear Editor:
Advanced glycation end products (AGEs) are generated by the Maillard reaction between an aldehyde group and an amino group of a protein. The resulting protein degeneration and inflammation are linked to both aging and hyperglycemia12. Numerous carbonyl compounds are present in vivo, including reducing sugars such as glucose or fructose and intermediates of glucose metabolism. Glyceraldehyde (GA), which is an intermediate product of both glycolysis and polyol metabolism, plays an important role in the pathogenesis of lifestyle-related diseases through the formation of glyceraldehyde-derived AGEs (Glycer-AGEs). For example, Glycer-AGEs contribute to microvascular complications of diabetes, i.e., retinopathy and nephropathy and the malignancy of cancer via receptor for AGEs (RAGE) signal transduction followed by enhancement of intercellular ROS production345. It is thus expected that the presence of Glycer-AGEs is highly related to intracellular metabolism in normal skin cells. However, the presence of Glycer-AGEs in epidermal cells, 95% of which are keratinocytes, has not been shown. We report an immunohistochemical detection of Glycer-AGEs in skin using a Glycer–AGE-specific antibody and the rates of AGE formation with GA and glyoxal (GO).
Skin samples were purchased from Biopredic International (Saint-Grégoire, France), fixed in 4% buffered paraformaldehyde, and embedded in paraffin. After deparaffinization and antigen retrieval by heating in a microwave in 10 mM sodium citrate buffer (pH 6), sections were washed in 0.1% phosphate-buffered saline (PBS) with Tween-20 for 30 min and prepared for immunohistochemistry6. Nonspecific staining was blocked by preincubation with 1% bovine serum albumin (BSA) in PBS for 1 h at room temperature. Skin sections were incubated with the anti-Glycer–AGE antibody (provided by Dr. Takeuchi, Kanazawa Medical University) at a 1:200 dilution in 1% BSA-PBS overnight at 4℃. For blocking peptide-treated samples, we used primary antibody incubated with a blocking peptide for 1 h at RT. Bound antibodies were visualized with Alexa 488-conjugated secondary antibody at a 1:200 dilution in 1% BSA-PBS for 1 h at RT, and hoechst33258 was added for nuclear counterstaining. All images were obtained using an IX71 microscope (Olympus, Tokyo, Japan).
GA and GO were used as the glycation inducer to assay AGE formation in vitro. Skin was removed from 7-week-old female hairless (Hos:HR-1) mice (Hoshino Experiment Animal Center, Ibaraki, Japan), cleaned of subcutaneous fat, and mounted in modified vertical diffusion cells. The cells had an effective diffusion area of 1.77 cm2 and a receptor compartment volume of 5.0 ml. For induction of glycation, the skin specimens were hydrated from the dermal side for 6 to 24 h with PBS containing either 10 mM or 50 mM GO or GA. The receptor fluid was maintained at 32℃, and continuously agitated with a magnetic stirrer bar. After induction, the skin was placed on a white board, and the color was measured using a CR-400 chromameter (Konica-Minolta, Tokyo, Japan). Data were expressed in the L*a*b* color space, and the b* was used as the yellow color value. The skin samples were then minced in 50% methanol and sonicated. Following centrifugation, AGEs were assayed in the supernatant by fluorescence with excitation at 365 nm and emission at 450 nm (SpectraMax M2e; Molecular Devices, Sunnyvale, CA, USA). To measure the modification rate of Lys, 10 mM or 50 mM of GO or GA was reacted with 10 mM of Lys in PBS at 37℃ for predetermined times. After hydrolysis in 6 N HCl for 8 h at 110℃, the concentration of modified Lys was measured by JLC-500 amino acid analyzer (JEOL Ltd., Tokyo, Japan).
The presence of Glycer-AGEs in the epidermis and dermis of human skin was demonstrated by immunohistochemistry, but they were not found in skin pretreated with blocking peptides. Glycer-AGEs were detected in stratum corneum and viable epidermis, and to a lesser extent, dermal cells. No significant differences between young and old skin were observed (Fig. 1).
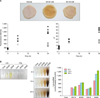
To describe the presence of Glycer-AGEs in epidermis, which has a turnover rate about 1 month, we compared the rates of AGE formation induced by GO and GA. The respective amounts of AGEs induced by GA, as indicated by fluorescence intensity and b* value, were 3.0 and 2.2 times higher than those of the AGEs induced by GO. Interestingly, the intensity and b* values induced by GO peaked at 6 h, whereas they continued to increase when induced by GA (Fig. 2A). Finally, when the basic amino acids Lys, Arg reacted with glycation inducer, only Lys:GA showed a strong yellowish tint (Fig. 2B). Consistent with this, the modification rate of Lys from 6 to 24 h with 50 mM GA was greater than that of GO (26 vs. 6 nmol/h) (Fig. 2B).
Glycer-AGEs were present in both epidermis and dermis. Because of the rapid turnover rate in the epidermis, dermal AGEs present in long-lived substrates in the ECM such as collagen or elastic fiber have been the focus of research for a long time. Kawabata et al.7 first reported the presence of AGEs in epidermis, the major one being Nε-(carboxymethyl)lysine (CML), derived from GO and they found that CML was localized primarily in epidermal keratin 10 and dermal ECM. However, we found that Glycer-AGEs were concentrated at both epidermal and dermal cells. These results suggest that the accumulation of Glycer-AGEs in skin strongly reflects alterations in intracellular metabolism caused by lifestyle-related conditions like hyperglycemia. Though we couldn't detect the difference in Glycer-AGEs accumulation between young and aged human skin, Glycer-AGEs may contribute to skin aging via upregulation of RAGE expression in hyperglycemic condition8. Furthermore, the faster increase in fluorescence intensity and b* value in GA-versus GO-glycated skin suggests that Glycer-AGEs accumulate at early stages of hyperglycemia, playing a pro-inflammatory role via RAGE signaling and contribute to degeneration of proteins. In addition, we found a strong relationship between a yellowish tint of the skin and faster Lys modification by GA. Further studies are required to elucidate the biological and physiological significance of Glycer-AGEs in skin, but our study suggests that they may influence normal human keratinocytes and fibroblasts by different mechanisms and accumulate to a different degree than GO-induced AGEs.
Figures and Tables
Fig. 1
Glyceraldehyde-derived advanced glycation end products (Glycer-AGEs) in sections of human skin from the abdominal area of individuals 28 and 63 years of age following immunohistochemical staining with anti-Glycer-AGEs antibody. Pretreatment with a blocking peptide resulted in significant loss of signal. Lower panels are shown with nuclear counterstaining. Bar=100 µm.
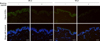
Fig. 2
(A) Macroscopic view of hairless mouse skin glycated using glyoxal (GO) or glyceraldehyde (GA). Rate of increase in advanced glycation end product (AGE) formation indicated by fluorescence intensity (excitation: 365 nm/emission: 450 nm) and b* value. Glycation induced by 50 mM of GO: ○, or GA: ▲. (B) Macroscopic view of glycated amino acids before hydrolysis (6 h) and after hydrolysis (6, 12, 24 h). Rate of modification of Lys as determined by amino acid analysis.
References
1. Gkogkolou P, Böhm M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol. 2012; 4:259–270.
2. Zhu P, Ren M, Yang C, Hu YX, Ran JM, Yan L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp Dermatol. 2012; 21:123–129.

3. Miura J, Yamagishi Si, Uchigata Y, Takeuchi M, Yamamoto H, Makita Z, et al. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with Type 1 diabetes. J Diabetes Complicat. 2003; 17:16–21.

4. Takino J, Yamagishi S, Takeuchi M. Cancer malignancy is enhanced by glyceraldehyde-derived advanced glycation end-products. J Oncol. 2010; 2010:739852.

5. Abe R, Shimizu T, Sugawara H, Watanabe H, Nakamura H, Choei H, et al. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J Invest Dermatol. 2004; 122:461–467.

6. Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med. 2000; 6:114–125.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download