Abstract
Nocardia species are aerobic, gram-positive, filamentous, partially acid-fast actinomycetes which are found worldwide in soil and decaying organic plant matter. When they infect human beings, they generally enter through the respiratory tract and then disseminate systemically. Rarely has a primary infection occurred as the result of direct inoculation. Isolation of Nocardia from clinical specimens and identification of species are difficult. But, with the introduction of new genetic technologies, reports of novel species of Nocardia have increased. We describe a case of cutaneous nocardiosis caused by Nocardia takedensis in an 87-year-old woman who was diagnosed by bacterial culture and 16S ribosomal RNA sequencing. N. takedensis has been described as a new species. This report describes the first clinical isolate of N. takedensis from a skin specimen in Korea.
Nocardia is an uncommon human pathogen infecting the lungs, skin, central nervous system, or other organs. It can present as a localized or disseminated infection123. In 2005, Nocardia takedensis was isolated from moat sediment and sludge around the Takeda shrine in Japan and reported as a novel species45. This species is closely related to N. beijingensis (98.1%~98.3%), N. brasiliensis (97.9%~98.0%), and N. tenerifensis (97.8%~97.9%) according to 16S ribosomal RNA (rRNA) sequencing5. We report a case of cutaneous nocardiosis caused by N. takedensis, which is the first report of an infection caused by this species in Korea.
An 87-year-old Korean woman presented with erythematous and swollen plaque on her left forearm. She was elderly and lived alone in a rural area adjacent to Busan. When spraying an insecticide to kill ants in her house, she had suffered an abrasion on the left forearm; the abrasion had been present for several weeks and had not been treated. The same area revealed tender erythematous with swollen plaque accompanied by pustules, fluctuated abscesses, ulcers, and crustiness (Fig. 1A, B). She had no other remarkable history besides hypertension.
Abnormal laboratory findings included a total white blood cell count of 19,300/mm3 with 89% neutrophils. No abnormal findings were found on a chest X-ray or computed tomography scan, electrocardiograph, or blood chemistry, electrolyte, and urinary analyses. A skin biopsy was performed for histopathologic examination.
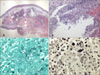
Histopathologic findings revealed by hematoxylin-eosin staining included intense edema in the upper dermis and dense multinodular pandermal and subcutaneous neutrophilic infiltration (Fig. 2A, B). Grocott-Gomori's methenamine silver staining identified thin, filamentous bacilli (Fig. 2C). On gram staining, the organism was seen to be a Gram-positive long rod with branching hypae (Fig. 2D). Periodic acid-Schiff (PAS) and PAS with diastase also revealed filamentous bacilli and Ziehl-Neelsen staining showed partial positive results of a cultured colony (Fig. 3A), but not in a tissue specimen. Fungal culture and mycobacterium tuberculosis-polymerase chain reaction were negative.
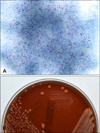
When pus culture was conducted, chalky-orange colored colonies formed on a blood agar plate 2 weeks after the start of incubation (Fig. 3B). Sequencing of 16S rRNA gene using a cultured colony was done for molecular identification. The isolated sequence was then compared with those of representative species of the genus Nocardia as classified in GenBank of the National Center for Biotechnology Information (NCBI). Our cultured isolate was identified as N. takedensis with 99.56% sequence similarity (3 nucleotide differences out of 688 nucleotides) to the strain WE30 (Fig. 4).
At the time the skin biopsy was taken (on the first hospital day), treatment by drip infusion of 2 g/day of ceftriaxone sodium was begun as an empirical therapy; treatment was continued for 16 days with improvement. After systemic disease was excluded and N. takedensis was identified, the patient was administered trimethoprim-sulfamethoxazole (TMP-SMX), 400/80 mg by mouth twice daily considering antimicrobial susceptibility and difficulties in securing an intravenous line. Treatment was maintained for 40 days until the patient recovered completely (Fig. 1C, D).
Nocardia is usually an “opportunistic pathogen,” with the majority of infections occurring in patients with immunosuppressive conditions. Up to one-third of patients with nocardiosis, however, are immnunocompetent. Nocardia are ubiquitous in the environment and can be found worldwide as saprophytic entities in fresh and salt water, soil, dust, and decaying vegetation6. Cutaneous manifestation of nocardiosis can be classified into primary cutaneous nocardiosis and disseminated disease. Primary cutaneous nocardiosis results from direct inoculation of pathogenic Nocardia. Disseminated disease is more common and usually occurs in the setting of primary pulmonary infection and underlying immunosuppression7. Primary cutaneous nocardiosis can be further divided into three major subtypes: lymphocutaneous disease, superficial cellulitis, and nocardial mycetoma. Cutaneous nocardiosis can result from traumatic injury to the skin that involves contamination with soil and resembles soft tissue infection caused by Staphylococcus aureus or streptococci; however, this form of nocardial disease usually follows an indolent course6. We supposed that the organism might have gained access via a contaminated superficial abrasion on the patient's forearm, thus formimg superficial cellulitis in our case.
Three cases of primary cutaneous nocardiosis caused by N. takedensis have been identified. The first case involved a 68-year-old woman in Taiwan who had chronic kidney disease and diabetes mellitus and presented with foot cellulitis. N. takedensis was isolated from a biopsy of foot tissue8. The second case was a healthy 33-year-old man in Mexico who presented with hemithoracic actinomycetoma9. The last case was a 68-year-old woman with a history of follicular lymphoma and myelodysplastic syndrome after allogenic peripheral blood stem cell transplantation who presented with painful lymphangitic erythematous papules on the left arm after suffering an abrasion on the left dorsal hand7. All patients were treated with oral TMP-SMX.
The genus Nocardia consists of distinctive gram-positive variably acid-fast, strictly aerobic bacteria that form branched aerial and substrate filaments which, as they age, fragment into pleomorphic rod-shaped or coccoid elements. The colonies with abundant aerial filamentous growths have a chalky white or cotton-ball appearance on blood agar plates and may resemble Streptomyces species or, superficially, even some fungi3. The organisms are rarely partially acid–fast with routine Ziehl-Neelsen stain. Actinomyces may appear morphologically similar to these organisms, but unlike Nocardia, they are not acid–fast and grow under anaerobic conditions. Most other fungal hyphal structures do not figure in the differential diagnosis of Nocardia as they have much thicker hyphal forms10.
Clinicians and pathologists should be aware that the Nocardia genus continues to undergo the addition of new species; more than 50 species have been identified to date. The species variability in general morphologic characteristics and acid fastness, which is caused by differences in cell wall mycolic acid composition and the type of stain used, makes identification and species differentiation challenging7. Classical identification based on colony morphology or morphological characteristics of Nocardia does not allow for differentiation between the numerous species. The new molecular methods based on the 16S rRNA gene are crucial for Nocardia identification11. The different species of Nocardia display variable in vitro antimicrobial susceptibility patterns, so management of nocardial infections must be individualized6.
TMP-SMX is active against most species; however, clinicians should note that N. otitidiscaviarum, N. nova, and N. farcinica have shown notable resistance and that up to 42% of Nocardia isolates submitted to the Centers for Disease Control and Prevention for antimicrobial susceptibility testing between 1995 and 2004 were resistant to TMP-SMX7. Alternative antimicrobial agents with activity against Nocardia include amikacin, imipenem, meropenem, ceftriaxone, cefotaxime, minocycline, moxifloxacin, levofloxacin, linezolid, tigecyclin, and amoxicillin-clavulanic acid. Combination therapy with imipenem and cefotaxime, amikacin and TMP-SMX, imipenem and TMP-SMX, amikacin and cefotaxime, or amikacin and imipenem may provide enhanced efficacy6. The suggested duration of therapy ranges from 6 weeks for minor infections to 1 year for severe systemic diseases12. In our case, the minimum inhibitory concentration ceftriaxone, amikacin and TMP-SMX were checked. The bacterial strain was susceptible to ceftriaxone and TMP-SMX.
In conclusion, the new molecular identification methods based on the 16S rRNA gene for Nocardia identification are crucial11. Because there are no optimal recommendations for treatment of all Nocardia species, physicians should use empirical combination therapy that may include TMP-SMX, ceftriaxone, and imipenem until susceptibility results are available. The authors recommend that identification and antimicrobial susceptibility testing be performed by a reference laboratory to help guide therapeutic decisions for all clinical Nocardia isolates13.
Figures and Tables
Fig. 1
Cutaneous nocardiosis from Nocardia takedensis infection. The left lower arm shows erythematous and edematous plaque, multiple ulcerations, abscesses, and black crusts. (A) Pretreatment lateral side, (B) pretreatment medial side, (C) posttreatment lateral side, (D) posttreatment medial side.
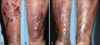
Fig. 2
Histopathologic findings. (A) Intense edema in the upper dermis and dense multinodular pandermal and subcutaneous neutrophilic infiltration (H&E, ×40). (B) Dense inflammatory infiltrates, with abscess formation, in the dermis (H&E, ×200). (C) Fine, filamentous branching bacilli (Grocott-Gomori's methenamine silver [GMS] stain, ×1,000). (D) Gram-positive rods with long, sinuous branches (Gram stain, ×1,000).
References
1. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006; 19:259–282.

2. Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J Clin Microbiol. 2003; 41:4497–4501.
4. Yamamura H, Hayakawa M, Nakagawa Y, Tamura T, Kohno T, Komatsu F, et al. Nocardia takedensis sp. nov., isolated from moat sediment and scumming activated sludge. Int J Syst Evol Microbiol. 2005; 55:433–436.

5. Japan MLJVTGJ. Takeda shrine. 2007-2012 [Internet]. MustLoveJapan;2014 Oct 29. Available from: http://www.mustlovejapan.com/subject/takeda_jinja/.
7. Chung E, Pulitzer MP, Papadopoulos EB, Papanicolaou GA, Babady NE, Marchetti MA. Lympahngitic papules caused by Nocardia takedensis. JAAD Case Rep. 2015; 1:126–128.
8. Liu WL, Lai CC, Ko WC, Chen YH, Tang HJ, Huang YL, et al. Clinical and microbiological characteristics of infections caused by various Nocardia species in Taiwan: a multicenter study from 1998 to 2010. Eur J Clin Microbiol Infect Dis. 2011; 30:1341–1347.

9. Kresch-Tronik NS, Carrillo-Casas EM, Arenas R, Atoche C, Del Río-Ávila C, Ochoa-Carrera LA, et al. First case of mycetoma associated with Nocardia takedensis. J Dermatol. 2013; 40:135–136.

10. Mathur S, Sood R, Aron M, Iyer VK, Verma K. Cytologic diagnosis of pulmonary nocardiosis: a report of 3 cases. Acta Cytol. 2005; 49:567–570.
11. Betrán A, Rezusta A, Lezcano MA, Villuendas MC, Revillo MJ, Boiron P, et al. First Spanish case of nocardiosis caused by Nocardia takedensis. J Clin Microbiol. 2009; 47:1918–1919.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download