1. Sala L, Bascones-Martínez A, Carrillo-de-Albornoz A. Impact of abutment material on peri-implant soft tissue color. An in vitro study. Clin Oral Investig. 2017; 21:2221–2233.

2. Sicilia A, Quirynen M, Fontolliet A, Francisco H, Friedman A, Linkevicius T, Lutz R, Meijer HJ, Rompen E, Rotundo R, Schwarz F, Simion M, Teughels W, Wennerberg A, Zuhr O. Long-term stability of peri-implant tissues after bone or soft tissue augmentation. Effect of zirconia or titanium abutments on peri-implant soft tissues. Summary and consensus statements. The 4th EAO Consensus Conference 2015. Clin Oral Implants Res. 2015; 26:148–152. PMID:
26385628.

3. Sailer I, Zembic A, Jung RE, Hämmerle CH, Mattiola A. Single-tooth implant reconstructions: esthetic factors influencing the decision between titanium and zirconia abutments in anterior regions. Eur J Esthet Dent. 2007; 2:296–310. PMID:
19655552.
4. Lops D, Bressan E, Parpaiola A, Sbricoli L, Cecchinato D, Romeo E. Soft tissues stability of cad-cam and stock abutments in anterior regions: 2-year prospective multicentric cohort study. Clin Oral Implants Res. 2015; 26:1436–1442. PMID:
25196805.

5. Park SE, Da Silva JD, Weber HP, Ishikawa-Nagai S. Optical phenomenon of peri-implant soft tissue. Part I. Spectrophotometric assessment of natural tooth gingiva and peri-implant mucosa. Clin Oral Implants Res. 2007; 18:569–574. PMID:
17655713.

6. Ioannidis A, Cathomen E, Jung RE, Fehmer V, Hüsler J, Thoma DS. Discoloration of the mucosa caused by different restorative materials - a spectrophotometric in vitro study. Clin Oral Implants Res. 2017; 28:1133–1138. PMID:
27452796.
7. Bittencourt TC, Ribeiro CG, Devito KL, Ferreira CF, Cagna DR, Picorelli NM. Zirconia abutment supporting all ceramic crowns in the esthetic zone: Interim results of a prospective study. Eur J Prosthodont Restor Dent. 2016; 24:23–30. PMID:
27039475.
8. Zembic A, Sailer I, Jung RE, Hämmerle CH. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin Oral Implants Res. 2009; 20:802–808. PMID:
19486077.

9. Degidi M, Artese L, Scarano A, Perrotti V, Gehrke P, Piattelli A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J Periodontol. 2006; 77:73–80. PMID:
16579706.

10. Nakamura K, Kanno T, Milleding P, Ortengren U. Zirconia as a dental implant abutment material: a systematic review. Int J Prosthodont. 2010; 23:299–309. PMID:
20617217.
11. Brodbeck U. The ZiReal Post: A new ceramic implant abutment. J Esthet Restor Dent. 2003; 15:10–23. PMID:
12638769.

12. Coray R, Zeltner M, Özcan M. Fracture strength of implant abutments after fatigue testing: A systematic review and a meta-analysis. J Mech Behav Biomed Mater. 2016; 62:333–346. PMID:
27239815.

13. Gehrke P, Johannson D, Fischer C, Stawarczyk B, Beuer F. In vitro fatigue and fracture resistance of one- and two-piece CAD/CAM zirconia implant abutments. Int J Oral Maxillofac Implants. 2015; 30:546–554. PMID:
26009905.

14. Garine WN, Funkenbusch PD, Ercoli C, Wodenscheck J, Murphy WC. Measurement of the rotational misfit and implant-abutment gap of all-ceramic abutments. Int J Oral Maxillofac Implants. 2007; 22:928–938. PMID:
18271374.
15. Ferrari M, Carrabba M, Vichi A, Goracci C, Cagidiaco MC. Influence of abutment color and mucosal thickness on soft tissue color. Int J Oral Maxillofac Implants. 2017; 32:393–399. PMID:
27525517.

16. Ferrari M, Tricarico MG, Cagidiaco MC, Vichi A, Gherlone EF, Zarone F, Sorrentino R. 3-year randomized controlled prospective clinical trial on different CAD-CAM implant abutments. Clin Implant Dent Relat Res. 2016; 18:1134–1141. PMID:
26988025.

17. Scarano A, Piattelli M, Vrespa G, Caputi S, Piattelli A. Bacterial adhesion on titanium nitride-coated and uncoated implants: an in vivo human study. J Oral Implantol. 2003; 29:80–85. PMID:
12760451.

18. Annunziata M, Oliva A, Basile MA, Giordano M, Mazzola N, Rizzo A, Lanza A, Guida L. The effects of titanium nitride-coating on the topographic and biological features of TPS implant surfaces. J Dent. 2011; 39:720–728. PMID:
21856369.

19. Vaz F, Cerqueira P, Rebouta L, Nascimento SMC, Alves E, Goudeau P, Rivière JP, Pischow K, de Rijk J. Structural, optical and mechanical properties of coloured TiNxOy thin films. Thin Solid Films [Internet]. Elsevier BV;2004. 447-8:p. 449–454. Available from:
http://dx.doi.org/10.1016/s0040-6090(03)01123-4.
20. Lops D, Stellini E, Sbricoli L, Cea N, Romeo E, Bressan E. Influence of abutment material on peri-implant soft tissues in anterior areas with thin gingival biotype: a multicentric prospective study. Clin Oral Implants Res. 2017; 28:1263–1268. PMID:
27699895.

21. Ferrari M, Cagidiaco MC, Garcia-Godoy F, Goracci C, Cairo F. Effect of different prosthetic abutments on peri-implant soft tissue. A randomized controlled clinical trial. Am J Dent. 2015; 28:85–89. PMID:
26087573.
22. Groessner-Schreiber B, Hannig M, Dück A, Griepentrog M, Wenderoth DF. Do different implant surfaces exposed in the oral cavity of humans show different biofilm compositions and activities? Eur J Oral Sci. 2004; 112:516–522. PMID:
15560835.

23. Kim YS, Shin SY, Moon SK, Yang SM. Surface properties correlated with the human gingival fibroblasts attachment on various materials for implant abutments: a multiple regression analysis. Acta Odontol Scand. 2015; 73:38–47. PMID:
25183254.

24. Chien CC, Liu KT, Duh JG, Chang KW, Chung KH. Effect of nitride film coatings on cell compatibility. Dent Mater. 2008; 24:986–993. PMID:
18177932.

25. Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996; 7:201–211. PMID:
9151584.

26. Mehl C, Kern M, Schütte AM, Kadem LF, Selhuber-Unkel C5. Adhesion of living cells to abutment materials, dentin, and adhesive luting cement with different surface qualities. Dent Mater. 2016; 32:1524–1535. PMID:
27717514.

27. Grössner-Schreiber B, Griepentrog M, Haustein I, Müller WD, Lange KP, Briedigkeit H, Göbel UB. Plaque formation on surface modified dental implants. An in vitro study. Clin Oral Implants Res. 2001; 12:543–551. PMID:
11737097.
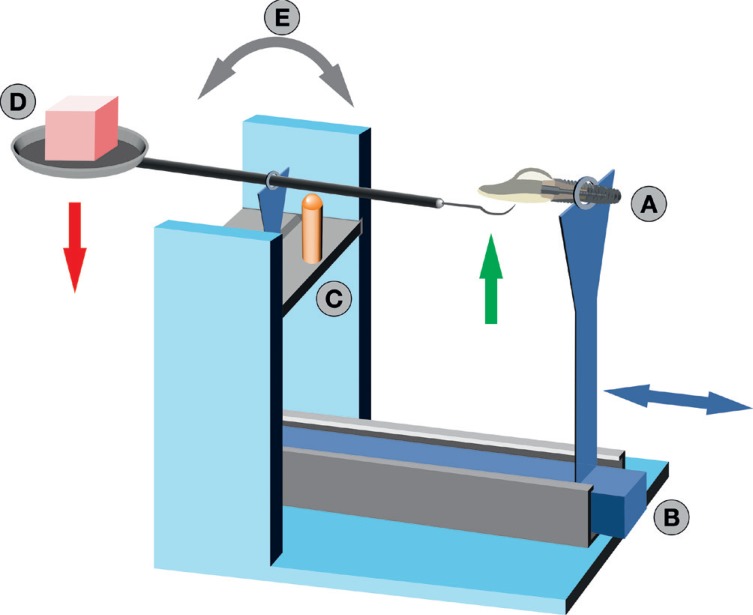
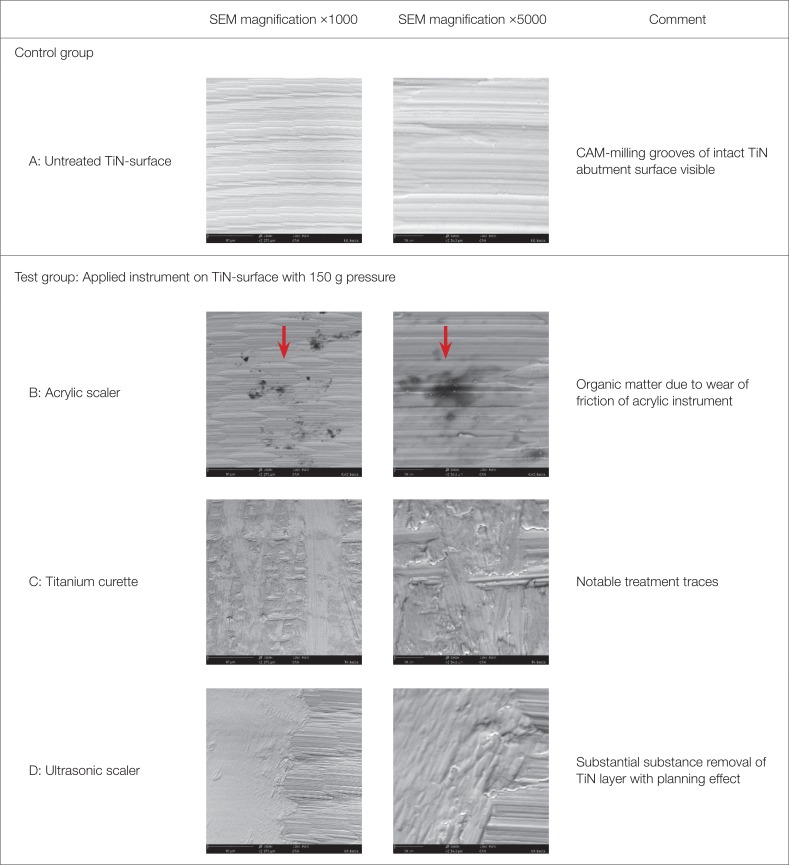
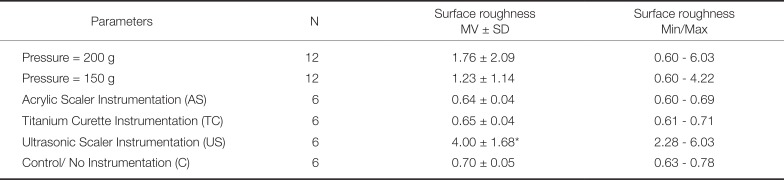
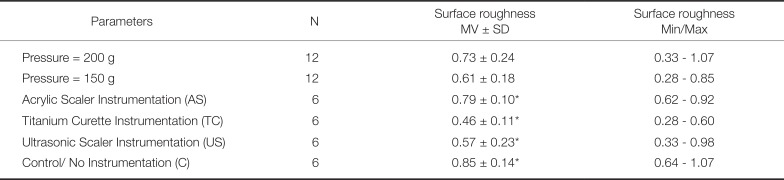
28. Mengel R, Meer C, Flores-de-Jacoby L. The treatment of uncoated and titanium nitride-coated abutments with different instruments. Int J Oral Maxillofac Implants. 2004; 19:232–238. PMID:
15101595.
29. Gehrke P, Tabellion A, Fischer C. Microscopical and chemical surface characterization of CAD/CAM zircona abutments after different cleaning procedures. A qualitative analysis. J Adv Prosthodont. 2015; 7:151–159. PMID:
25932314.

30. Rompen E, Domken O, Degidi M, Pontes AE, Piattelli A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res. 2006; 17:55–67. PMID:
16968382.

31. Louropoulou A, Slot DE, Van der Weijden F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: a systematic review. Clin Oral Implants Res. 2014; 25:1149–1160. PMID:
23834327.

32. Schwarz F, Papanicolau P, Rothamel D, Beck B, Herten M, Becker J. Influence of plaque biofilm removal on reestablishment of the biocompatibility of contaminated titanium surfaces. J Biomed Mater Res A. 2006; 77:437–444. PMID:
16444683.

33. Yang SM, Park JB, Ko Y. Use of confocal microscopy for quantification of plastic remnants on rough titanium after instrumentation and evaluation of efficacy of removal. Int J Oral Maxillofac Implants. 2015; 30:519–525. PMID:
26009902.

34. Schmage P, Kahili F, Nergiz I, Scorziello TM, Platzer U, Pfeiffer P. Cleaning effectiveness of implant prophylaxis instruments. Int J Oral Maxillofac Implants. 2014; 29:331–337. PMID:
24683558.

35. Schmidt KE, Auschill TM, Heumann C, Frankenberger R, Eick S, Sculean A, Arweiler NB. Influence of different instrumentation modalities on the surface characteristics and biofilm formation on dental implant neck, in vitro. Clin Oral Implants Res. 2017; 28:483–490. PMID:
27000771.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download