1. Marx RE. Clinical application of bone biology to mandibular and maxillary reconstruction. Clin Plast Surg. 1994; 21:377–392. PMID:
7924135.

2. Hoexter DL. Bone regeneration graft materials. J Oral Implantol. 2002; 28:290–294. PMID:
12498538.

3. Quattlebaum JB, Mellonig JT, Hensel NF. Antigenicity of freeze-dried cortical bone allograft in human periodontal osseous defects. J Periodontol. 1988; 59:394–397. PMID:
2455783.
4. Sogal A, Tofe AJ. Risk assessment of bovine spongiform encephalopathy transmission through bone graft material derived from bovine bone used for dental applications. J Periodontol. 1999; 70:1053–1063. PMID:
10505809.

5. Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002; 25:s571–s578. PMID:
12038844.

6. Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004; 25:987–994. PMID:
14615163.

7. Kondo N, Ogose A, Tokunaga K, Ito T, Arai K, Kudo N, Inoue H, Irie H, Endo N. Bone formation and resorption of highly purified beta-tricalcium phosphate in the rat femoral condyle. Biomaterials. 2005; 26:5600–5608. PMID:
15878364.
8. Gholami GA, Tehranchi M, Kadkhodazadeh M, Moghadam AA, Ghanavati F, Ardakani MRT, Aghaloo M, Mashhadiabbas F. Histologic and histomorphometric evaluation of bone substitutes in experimental defects. Res J Biol Sci. 2010; 5:465–469.

9. Bystedt H, Rasmusson L. Porous titanium granules used as osteoconductive material for sinus floor augmentation: a clinical pilot study. Clin Implant Dent Relat Res. 2009; 11:101–105. PMID:
18657154.

10. Al-Sabbagh M. Implants in the esthetic zone. Dent Clin North Am. 2006; 50:391–407. PMID:
16818022.

11. Wenz HJ, Bartsch J, Wolfart S, Kern M. Osseointegration and clinical success of zirconia dental implants: a systematic review. Int J Prosthodont. 2008; 21:27–36. PMID:
18350943.
12. Sollazzo V, Pezzetti F, Scarano A, Piattelli A, Bignozzi CA, Massari L, Brunelli G, Carinci F. Zirconium oxide coating improves implant osseointegration in vivo. Dent Mater. 2008; 24:357–361. PMID:
17640724.

13. Brüll F, van Winkelhoff AJ, Cune MS. Zirconia dental implants: a clinical, radiographic, and microbiologic evaluation up to 3 years. Int J Oral Maxillofac Implants. 2014; 29:914–920. PMID:
25032772.

14. Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005; 7:S13–S20. PMID:
16137083.

15. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999; 20:1–25. PMID:
9916767.

16. Gredes T, Kubasiewicz-Ross P, Gedrange T, Dominiak M, Kunert-Keil C. Comparison of surface modified zirconia implants with commercially available zirconium and titanium implants: a histological study in pigs. Implant Dent. 2014; 23:502–507. PMID:
25025856.
17. Depprich R, Zipprich H, Ommerborn M, Mahn E, Lammers L, Handschel J, Naujoks C, Wiesmann HP, Kübler NR, Meyer U. Osseointegration of zirconia implants: an SEM observation of the bone-implant interface. Head Face Med. 2008; 4:25. PMID:
18990214.

18. Garvie RC, Hannink RH, Pascoe RT. Ceramic steel? Nature. 1975; 258:703–704.

19. Pae A, Lee H, Kim HS, Baik J, Woo YH. Cellular attachment and gene expression of osteoblast-like cells on zirconia ceramic surfaces. J Korean Acad Prosthodont. 2008; 46:227–237.
20. Guazzato M, Quach L, Albakry M, Swain MV. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. J Dent. 2005; 33:9–18. PMID:
15652163.

21. Kim YK, Kim SG, Kim BS, Jeong KI. Resorption of bone graft after maxillary sinus grafting and simultaneous implant placement. J Korean Assoc Oral Maxillofac Surg. 2014; 40:117–122. PMID:
25045638.

22. Silva NR, Sailer I, Zhang Y, Coelho PG, Guess PC, Zembic A, Kohal RJ. Performance of zirconia for dental healthcare. Materials. 2010; 3:863–896.

23. Orentlicher G, Goldsmith D, Abboud M. Computer-guided planning and placement of dental implants. Atlas Oral Maxillofac Surg Clin North Am. 2012; 20:53–79. PMID:
22365430.

24. Gahleitner A, Watzek G, Imhof H. Dental CT: imaging technique, anatomy, and pathologic conditions of the jaws. Eur Radiol. 2003; 13:366–376. PMID:
12599003.

25. Xu H, Shimizu Y, Asai S, Ooya K. Experimental sinus grafting with the use of deproteinized bone particles of different sizes. Clin Oral Implants Res. 2003; 14:548–555. PMID:
12969358.

26. Klawitter JJ, Hulbert SF. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J Biomed Mater Res. 1971; 5:161–229.

27. Huh JB, Jung DH, Kim JS, Shin SW. Effects of different sizes of Hydroxyapatite/β-Tricalcium phosphate particles on vertical bone augmentation. J Korean Acad Prosthodont. 2010; 48:259–265.

28. Song YG, Cho IH. Characteristics and osteogenic effect of zirconia porous scaffold coated with β-TCP/HA. J Adv Prosthodont. 2014; 6:285–294. PMID:
25177472.

29. Langhoff JD, Voelter K, Scharnweber D, Schnabelrauch M, Schlottig F, Hefti T, Kalchofner K, Nuss K, von Rechenberg B. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: a study in sheep. Int J Oral Maxillofac Surg. 2008; 37:1125–1132. PMID:
18977118.

30. Scarano A, Di Carlo F, Quaranta M, Piattelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003; 29:8–12. PMID:
12614079.

31. Kohal RJ, Weng D, Bächle M, Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004; 75:1262–1268. PMID:
15515343.

32. Pilathadka S, Vahalová D, Vosáhlo T. The Zirconia: a new dental ceramic material. An overview. Prague Med Rep. 2007; 108:5–12.
33. Roberts EW, Poon LC, Smith RK. Interface histology of rigid endosseous implants. J Oral Implantol. 1986; 12:406–416. PMID:
3470526.
34. Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS, Choi SH. Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal Implant Sci. 2010; 40:180–187. PMID:
20827327.

35. Becker W, Becker BE, Handelsman M, Ochsenbein C, Albrektsson T. Guided tissue regeneration for implants placed into extraction sockets: a study in dogs. J Periodontol. 1991; 62:703–709. PMID:
1753323.

36. Buser D, Hoffmann B, Bernard JP, Lussi A, Mettler D, Schenk RK. Evaluation of filling materials in membrane--protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin Oral Implants Res. 1998; 9:137–150. PMID:
10530128.

37. Gotfredsen K, Nimb L, Hjørting-Hansen E. Immediate implant placement using a biodegradable barrier, polyhydroxybutyrate-hydroxyvalerate reinforced with polyglactin 910. An experimental study in dogs. Clin Oral Implants Res. 1994; 5:83–91. PMID:
7918913.

38. Hämmerle CH, Jung RE. Bone augmentation by means of barrier membranes. Periodontol 2000. 2003; 33:36–53. PMID:
12950840.

39. LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med. 2003; 14:201–209. PMID:
15348465.
40. Kim YK, Yun PY, Lim SC, Kim SG, Lee HJ, Ong JL. Clinical evaluations of OSTEON as a new alloplastic material in sinus bone grafting and its effect on bone healing. J Biomed Mater Res B Appl Biomater. 2008; 86:270–277. PMID:
18161818.

41. Branemark PI, Zarb GA, Albrektsson T. Tissue integrated prostheses: Osseointegration in clinical dentistry. 1st ed. Quintessence Pub Co;1985. p. 221–229.
42. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–170. PMID:
7246093.
43. Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977; 16:1–132. PMID:
356184.
44. Wohlfahrt JC, Monjo M, Rønold HJ, Aass AM, Ellingsen JE, Lyngstadaas SP. Porous titanium granules promote bone healing and growth in rabbit tibia peri-implant osseous defects. Clin Oral Implants Res. 2010; 21:165–173. PMID:
19912270.

45. Thor A. Porous titanium granules and blood for bone regeneration around dental implants: Report of four cases and review of the literature. Case Rep Dent. 2013; 2013:410515. PMID:
23533827.

46. Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA. Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone. 2009; 45:1104–1116. PMID:
19651256.

47. Ejima K, Omasa S, Motoyoshi M, Arai Y, Kai Y, Amemiya T, Yamada H, Honda K, Shimizu N. Influence of metal artifacts on in vivo micro-CT for orthodontic mini-implants. J Oral Sci. 2012; 54:55–59. PMID:
22466887.

48. Kim HW, Georgiou G, Knowles JC, Koh YH, Kim HE. Calcium phosphates and glass composite coatings on zirconia for enhanced biocompatibility. Biomaterials. 2004; 25:4203–4213. PMID:
15046910.

49. Kim HW, Lee SY, Bae CJ, Noh YJ, Kim HE, Kim HM, Ko JS. Porous ZrO2 bone scaffold coated with hydroxyapatite with fluorapatite intermediate layer. Biomaterials. 2003; 24:3277–3284. PMID:
12763455.

50. Kim HW, Kim HE, Salih V, Knowles JC. Dissolution control and cellular responses of calcium phosphate coatings on zirconia porous scaffold. J Biomed Mater Res A. 2004; 68:522–530. PMID:
14762932.




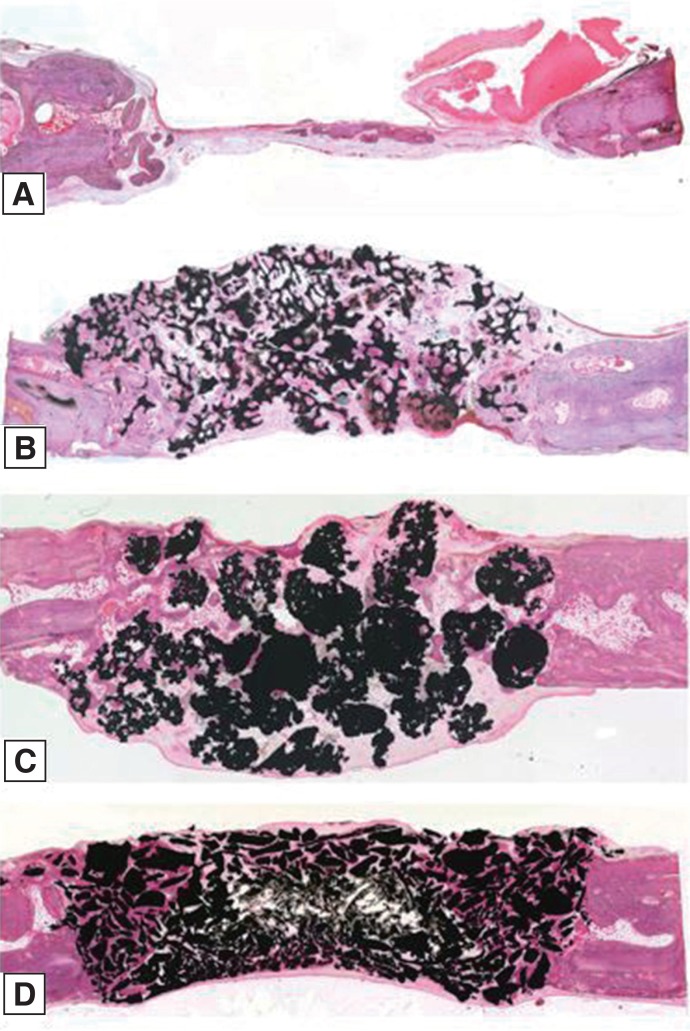
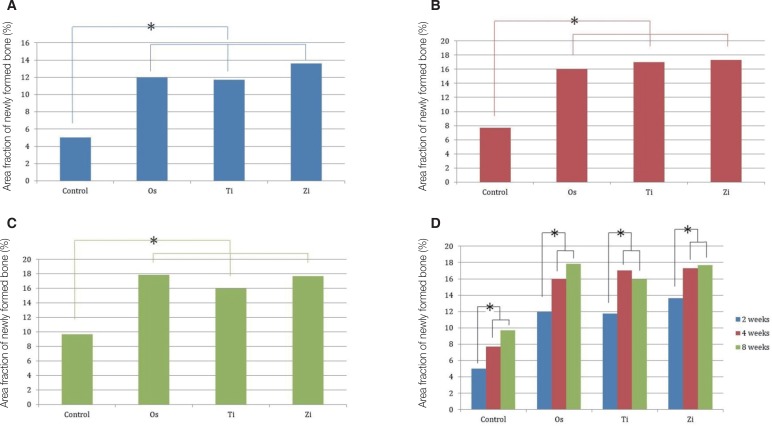

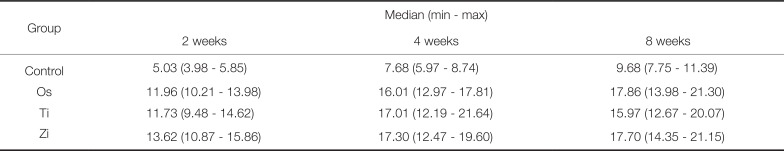
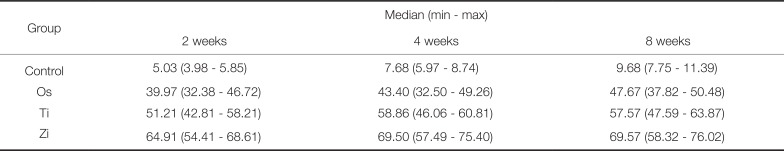




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download