Abstract
Purpose
There has been little research regarding the effectiveness of oseltamivir for influenza B infections. We sought to identify the different clinical manifestations between patients treated with and without oseltamivir. Methods: We retrospectively studied the medical records of 72 inpatients or outpatients from two medical centers diagnosed with influenza B infections by either a rapid antigen test or multiplex reverse transcriptase PCR between January 2012 and July 2012. We compared gender, age, past medical history, admission period, total fever duration, fever duration after hospitalization, post-oseltamivir medication peak temperature, laboratory test, chest X-ray, antibiotic medication, and the presence of concomitant viral or bacterial infections. Results: The number of subjects in our study was 72 who were diagnosed with influenza B pneumonia, acute bronchitis, acute bronchiolitis, croup, and mean age was 3.6±2.8 year old. The demographic characteristics and clinical manifestations of oseltamivir and the non-oseltamivir groups, including hospitalization period (4.18±2.10 vs 4.79±1.49 days, P=.17) and total fever duration (5.32±2.07 vs 6.41±3.25 days, P=.09), demonstrated no significant differences. Notably, the oseltamivir group did have significantly reduced usage of antibiotic treatment than the non-oseltamivir group (P=.04). When we limited our patient group to patients under the age of three, similar results were seen. The group prescribed oseltamivir within 48 hours of fever onset had less antibiotic usage, in addition to a shorter fever duration. Conclusion: Oseltamivir appeared to have no benefit in improving the clinical course. However, if it is prescribed within the first 48 hours of symptoms, it may be more effective.
Go to : 
References
2. Burnham AJ, Baranovich T, Govorkova EA. Neuraminidase inhibitors for influenza B virus infection: efficacy and resistance. Antiviral Res. 2013; 100:520–34.

3. Lee NY, Park JH, Kim GH, Jung JH, Cho KS, Kim SM. Viral etiology and clinical pattern of acute lower respiratory tract infection in children (Busan Area in 2002). Korean J Pediatr Infect Dis. 2003; 10:87–94.
4. Kwon JH, Chung YH, Lee NY, Chung EH, Ahn KM, Lee SI. An epidemiological study of acute viral lower respiratory tract infections in hospitalized children from 2002 to 2006 in Seoul, Korea. Pediatr Allergy Respir Dis (Korea). 2008; 18:26–36.
5. Kim MS, Sung HW, Bae EY, Han SB, Jeong DC, Kang JH. The clinical characteristics of influenza B infection during the 2011–2012 influenza season. Korean J Pediatr Infect Dis. 2013; 20:89–97.

6. Bicer S, Giray T, Col D, Erdag GC, Vitrinel A, Gurol Y, et al. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr. 2013; 39:22.

7. Kang TG, Kim MJ, Kim BG, An HS, Yun HJ, Choi EJ, et al. Comparisons of clinical features among influenza A (H1N1) and seasonal influenza A and B during 2009 to 2010 at a single institution. Pediatr Allergy Respir Dis (Korea). 2011; 21:269–76.

8. Kim SG, Hwang YH, Shin YH, Kim SW, Jung WS, Kim SM, et al. Occurrence and characterization of oseltamivir-resistant influenza virus in children between 2007–2008 and 2008–2009 seasons. Korean J Pediatr. 2013; 56:165–75.

9. Leang SK, Deng YM, Shaw R, Caldwell N, Iannello P, Komadina N, et al. Influenza antiviral resistance in the Asia-Pacific region during 2011. Antiviral Res. 2013; 97:206–10.

10. Sugaya N, Mitamura K, Yamazaki M, Tamura D, Ichikawa M, Kimura K, et al. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis. 2007; 44:197–202.

11. Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Care-wicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000; 355:1845–50.
12. Seo ES, Park GH, Kim SM, Kim SW, Jung WS, Cho KS, et al. Oseltamivir efficacy, side effects, and safety in children with influenza. Korean J Pediatr. 2010; 53:56–66.

13. Roche Korea. Information on product: Tamiflu leaflet [Internet]. Seoul: Korea R;c2013. [cited 2013]. Available from:. http://www.roche.co.kr/file/Tamiflu. %20leaflet. pdf.
14. Kim SH, Park CH, Huh K, Shim GH, Kim HB, You SJ, et al. Comparison of clinical manifestation and laboratory findings between H1N1 and influenza B infection. Pediatr Allergy Respir Dis. 2012; 22:64–70.

15. Nah SY, Park SE, Park JY, Lee HJ. Epidemiology of influenza virus over 8 years (1990–1998) in Seoul, Korea. Korean J Infect Dis. 1999; 31:210–6.
16. Kawai N, Ikematsu H, Iwaki N, Maeda T, Satoh I, Hiro-tsu N, et al. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clin Infect Dis. 2006; 43:439–44.

17. Mitamura K, Sugaya N, Nirasawa M, Shinjoh M, Takeuchi Y. Effectiveness of oseltamivir treatment against influenza type A and type B infection in children. Kansenshogaku zasshi. 2002; 76:946–52.

18. Whitley RJ, Hayden FG, Reisinger KS, Young N, Dut-kowski R, Ipe D, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis. 2001; 20:127–33.

19. Heinonen S, Silvennoinen H, Lehtinen P, Vainionpaa R, Vahlberg T, Ziegler T, et al. Early oseltamivir treatment of influenza in children 1–3 years of age: a randomized controlled trial. Clin Infect Dis. 2010; 51:887–94.

20. Fujisaki S, Takashita E, Yokoyama M, Taniwaki T, Xu H, Kishida N, et al. A single E105K mutation far from the active site of influenza B virus neuraminidase contributes to reduced susceptibility to multiple neuraminidase-inhibitor drugs. Biochem Biophys Res Commun. 2012; 429:51–6.

22. Korea Centers for Disease Control and Prevention. Korean influenza surveillance report, 2011–2012. Public Health Weekly Report. 2012; 5:873–81.
Go to : 
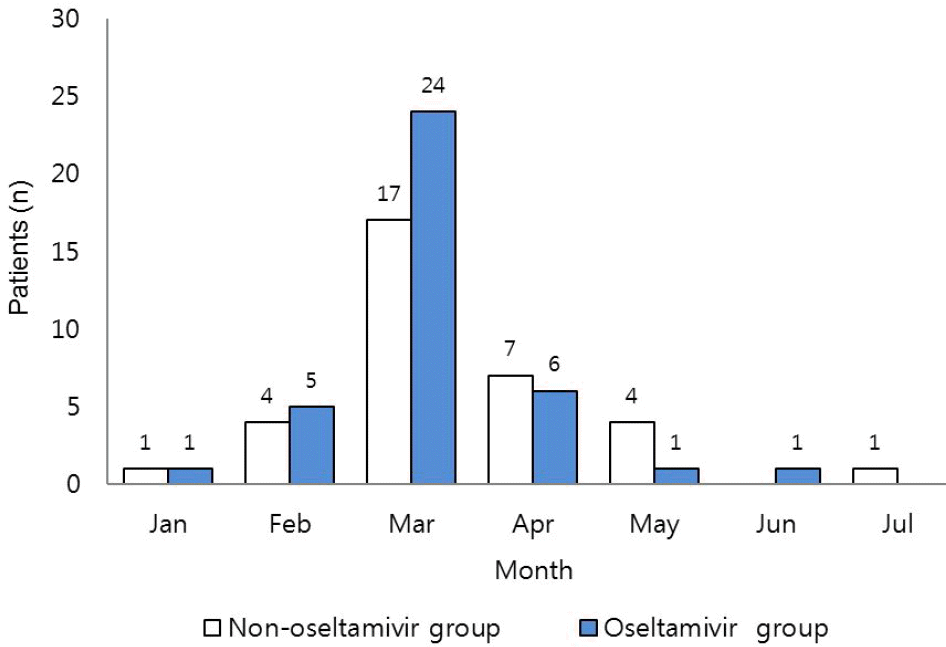 | Fig. 1.Monthly Distribution of Children with Influenza B Infections from January, 2012 to July, 2012. |
Table 1.
Comparison of Demographic Characteristics and Clinical Manifestations between Study Groups
| Characteristics | Total (n=72) | Total patients (n=72) | ||
|---|---|---|---|---|
| Non-oseltamivir∗(n=34) | Oseltamivir†(n=38) | P-value | ||
| Gender (male/female) | 37/35 | 18/16 | 19/19 | 0.80 |
| Age (years) | 3.6±2.78 | 3.03±2.34 | 4.11±3.06 | 0.10 |
| Daycare center (yes/no) | 45/13 | 23/4 | 22/9 | 0.31 |
| Past medical history‡ (yes/no) | 16/56 | 4/30 | 12/26 | 0.05 |
| Influenza vaccination (yes/no) | 24/38 | 11/17 | 13/21 | 1.00 |
| Cough (yes/no) | 67/5 | 31/3 | 36/2 | 0.66 |
| Sore throat (yes/no) | 24/45 | 11/21 | 13/24 | 0.95 |
| Rhinitis (yes/no) | 49/21 | 22/11 | 27/10 | 0.57 |
| GI Sxʃ (yes/no) | 28/44 | 11/23 | 17/21 | 0.28 |
| Admission (yes/no)∥ | 67/5 | 34/0 | 33/5 | 0.06 |
| Admission period (days) | 4.47±1.85 | 4.79±1.49 | 4.18±2.10 | 0.17 |
| Diagnostic method (Rapid antigen test/PCR) | 51/21 | 19/15 | 32/6 | 0.01 |
| Peak BT¶ (℃) | 39.5±0.69 | 39.58±0.78 | 39.40±0.56 | 0.29 |
| Antibiotics (yes/no) | 50/22 | 28/6 | 22/16 | 0.04 |
| Time lag days∥ | 3.5±2.57 | 3.79±3.25 | 3.24±1.76 | 0.38 |
| Fever duration after admission (days) | 2.6±1.51 | 2.71±1.38 | 2.37±1.59 | 0.38 |
| Total fever duration (days) | 5.87±2.86 | 6.41±3.25 | 5.32±2.07 | 0.09 |
| C-reactive protein (mg/dL) | 0.67 (0.35–2.82)∗∗ | 0.85 (0.55–3.41)∗∗ | 0.46 (0.03–1.94)∗∗ | 0.04 |
Table 2.
Comparison between Timing of Oseltamivir Administration
| Variables | Non-oseltamivir∗(n=36) | Within 48 hrs†(n=10) | After 48 hrs‡(n=26) | P-value |
|---|---|---|---|---|
| Gender (male/female) | 19/17 | 5/5 | 13/13 | 0.97 |
| Age (years) | 3.06±2.31 | 3.70±2.58 | 4.31±3.32 | 0.22 |
| Influenza vaccination | 11/18 | 2/5 | 11/15 | 0.42 |
| Cough (yes/no) | 29/3 | 7/1 | 23/0 | 0.28 |
| Sore throat (yes/no) | 9/23 | 3/5 | 9/14 | 0.67 |
| Rhinitis (yes/no) | 21/11 | 5/3 | 18/5 | 0.54 |
| GI Sxʃ (yes/no) | 11/21 | 3/5 | 12/11 | 0.41 |
| Admission (yes/no) | 35/1 | 10/0 | 22/4 | 0.10 |
| Admission period (days) | 4.61±1.68 | 5.10±1.37 | 4.04±2.18 | 0.25 |
| Peak BT¶ (℃) | 39.64±0.78 | 39.00±0.61 | 39.58±0.52 | 0.09 |
| Antibiotics (yes/no) | 30/6 | 2/8 | 18/8 | 0.00 |
| Time lag days∥ | 3.75±3.19 | 2.00±1.33 | 3.73±1.66 | 0.14 |
| Fever duration after admission (days) | 2.81±1.39 | 2.14±1.95 | 2.44±1.54 | 0.51 |
| Total fever duration (days) | 6.52±3.44 | 3.88±1.81 | 5.68±1.77 | 0.06 |
| C-reactive protein (mg/dL) | 0.85 (0.55–3.41)∗∗ | 0.30 (0.00–1.13)∗∗ | 1.25 (0.31–2.53)∗∗ | 0.28 |
Values are presented as mean±standard deviation or number.∗Children with influenza B not treated with oseltamivir.
† Children with influenza B given their first dose of oseltamivir within 48 hours from the onset of fever.
Table 3.
Demographic Characteristics and Clinical Manifestations in Children Under 3 Years of Age with Influenza B Infections (n=44)
| Characteristic | Non-oseltamivir group∗(n=24) | Oseltamivir group†(n=20) | P-value |
|---|---|---|---|
| Gender (male/female) | 12/12 | 10/10 | 1.00 |
| Age (years) | 1.83±0.82 | 1.80±0.95 | 0.80 |
| Daycare Center (yes/no) | 17/4 | 8/6 | 0.15 |
| Past medical history‡ (yes/no) | 1/23 | 4/16 | 0.16 |
| Influenza vaccination (yes/no) | 8/11 | 8/10 | 1.00 |
| Admission (yes/no) | 24/0 | 17/3 | 0.09 |
| Admission period (days) | 4.88±1.45 | 4.25±2.38 | 0.57 |
| Peak BT∫ (℃) | 39.62±0.87 | 39.40±0.38 | 0.66 |
| Antibiotics (yes/no) | 18/6 | 9/11 | 0.06 |
| Time lag days∥ | 4.25±3.29 | 3.10±1.94 | 0.25 |
| Fever duration after admission (days) | 2.95±1.23 | 2.75±1.57 | 0.65 |
| Total fever duration (days) | 6.58±3.28 | 5.74±2.16 | 0.82 |
| C-reactive protein (mg/dL) | 0.69 (0.52–3.16)∗∗ | 0.35 (0.00–1.78)∗∗ | 0.01 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download