Abstract
Purpose
This study was performed to assess the clinical and epidemiological changes after the introduction of the rotavirus vaccine in Korea, as well as to determine the efficacy of the rotavirus vaccine among hospitalized rotaviral gastroenteritis patients over the past two years. Methods: We analyzed yearly and seasonal patterns of 1,165 inpatients who were hospitalized for rotaviral gastroenteritis under the age of 5 years between 2006 and 2013. We also conducted a survey among 460 gastroenteritis patients who were hospitalized between 2012 and 2013 regarding the rotavirus vaccination and the symptoms of gastroenteritis. Among those individuals surveyed, clinical indices were analyzed for 124 patients who were tested positive for the rotavirus antigen. Results: The incidence of Rotaviral gastroenteritis have decreased significantly by year 2010. After the introduction and widespread dissemination of the rotavirus vaccine, the onset of the disease and the seasonal peak have been delayed. Overall, the vaccinated group showed a lower rate of positivity than the unvaccinated group. Among the hospitalized rotaviral gastroenteritis patients, the vaccinated group had a shorter hospitalization period, less severe clinical symptoms of gastroenteritis, and better laboratory test results. Conclusions: After introduction of the rotavirus vaccine in Korea, there were two main trends observed: 1) the overall level of disease incidence was reduced; 2) the severity of rotaviral gastroenteritis cases also decreased. Based on this data, more children should receive vaccination in order to prevent the rotavirus infection and decrease the severity of rotaviral gastroenteritis.
Go to : 
References
1. Bern C, Martines J, Zoysa I, Glass RI. The magnitude of the global problem of diarrheal disease: a ten-year update. Bull World Health Organ. 1992; 70:705–14.
2. Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009; 58:1–25.
3. Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003; 9:565–72.

4. Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004; 78:10213–20.

5. World Health Organization. Rotavirus vaccines: WHO position Paper. Wkly Epidemiol Rec. 1999; 74:33–40.
6. Nakagomi O, Cunliffe NA. Rotavirus vaccines: entering a new stage of deployment. Curr Opin Infect Dis. 2007; 20:501–7.

7. Heaton PM, Goveia MG, Miller JM, Offit P, Clark HF. Development of a pentavalent rotavirus vaccine against prevalent serotypes of rotavirus gastroenteritis. J Infect Dis. 2005; 192:S17–21.

9. Desai R, Oliveira LH, Parashar UD, Lopman B, Tate JE, Patel MM. Reduction in morbidity and mortality from childhood diarrhoeal disease after species A rotavirus vaccine introduction in Latin America – a review. Mem Inst Oswaldo Cruz. 2011; 106:907–11.

10. Tate JE, Cortese MM, Payne DC, Curns AT, Yen C, Esposito DH, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011; 30:S56–60.
11. Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatr Infect Dis J. 2011; 30:S25–9.

12. Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhoea in Mexico. N Engl J Med. 2010; 362:299–305.
13. Lanzieri TM, Costa I, Shafi FA, Cunha MH, OrtegaBarria E, Linhares AC, et al. Trends in hospitalizations from all-cause gastroenteritis in children younger than 5 years of age in Brazil before and after human rotavirus vaccine introduction, 1998–2007. Pediatr Infect Dis J. 2010; 29:673–5.

14. Lanzieri TM, Linhares AC, Costa I, Kolhe DA, Cunha MH, Ortega-Barria E, et al. Impact of rotavirus vaccination on childhood deaths from diarrhea in Brazil. Int J Infect Dis. 2011; 15:e206–10.

15. Sá fadi MA, Berezin EN, Munford V, Almeida FJ, de Moraes JC, Pinheiro CF, et al. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in Sã o Paulo, Brazil. Pediatr Infect Dis J. 2010; 29:1019–22.
16. Choi UY, Lee SY, Ma SH, Jang YT, Kim YJ, Kim HM, et al. Epidemiological changes in rotavirus gastroenteritis in children under 5 years of age after the introduction of rotavirus vaccines in Korea. Eur J Pediatr. 2013; 172:947–52.
17. Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006; 55:1–13.
18. Tate JE, Panozzo CA, Payne DC, Patel MM, Cortese MM, Fowlkes AL, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009; 124:465–71.

19. Ruiz-Palacios GM, Pé rez-Schael I, Velá zquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006; 354:11–22.

20. Kirkwood CD, Boniface K, Barnes GL, Bishop RF. Distri-bution of rotavirus genotypes after introduction of rotavirus vaccines, Rotarix® and RotaTeq®, into the National Immunization Program of Australia. Pediatr Infect Dis J. 2011; 30:S48–53.
21. Korea Centers for Disease Control and Prevention. 2012 Korea national immunization survey. Available at. http://www.cdc.go.kr.
Go to : 
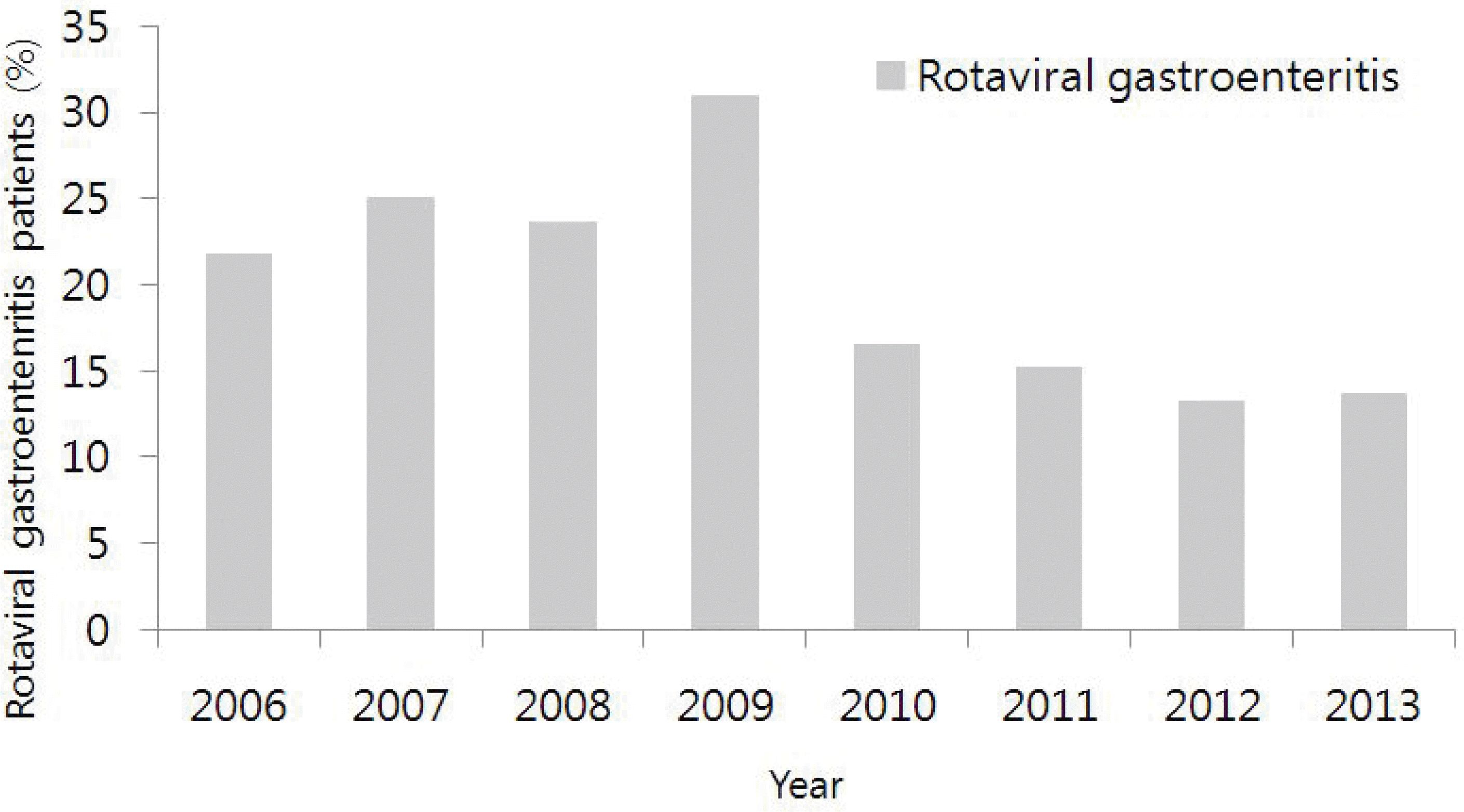 | Fig. 1.Annual percentage of rotaviral gastroenteritis patients out of all gastro-enteritis patients (2006–2013). |
Table 1.
Baseline Demographic and Clinical Comparison between the Vaccinated Group and Unvaccinated Group for Rotaviral Gastroenteritis
| Vaccinated (n=52) | Unvaccinated (n=72) | P | |
|---|---|---|---|
| Age (months) | 23.8±14.4 | 30.2±16.3 | 0.03 |
| Sex ratio (M:F) | 60:40 | 56:44 | 0.65 |
| Hospitalization (d) | 4.1±1.2 | 5.0±1.7 | <0.001 |
| Fever (n, %)∗ | 30 (57.7%) | 61 (84.7%) | <0.001 |
| Vomit (n, %) | 43 (82.7%) | 69 (95.8%) | 0.02 |
| Diarrhea ≥5 times/d (n, %)† | 16 (30.8%) | 35 (48.6%) | 0.046 |
Table 2.
Laboratory Results in the Vaccinated Group and Unvaccinated Group for Rotaviral Gastroenteritis
| Vaccinated (n=52) | Unvaccinated (n=72) | P | |
|---|---|---|---|
| WBC (/µ L) | 11,046±5,044 | 11,969±6,801 | 0.41 |
| Neutrophil (/µ L) | 6,482±4,103 | 8,279±6,094 | 0.07 |
| Lymphocyte (/µ L) | 3,407±2,324 | 2,621±2,606 | 0.09 |
| ESR (mm/hr) | 12.9±9.6 | 10.2±10.1 | 0.14 |
| CRP (mg/L) | 12.6±25.4 | 13.4±18.0 | 0.83 |
| Glucose (mg/dL) | 79.1±17.2 | 75.9±22.3 | 0.38 |
| Hypoglycemia (n, %)∗ | 6 (11.5%) | 18 (25.0%) | 0.06 |
| Uric acid (mg/dL) | 6.2±2.5 | 7.3±2.7 | 0.03 |
| Hyperuricemia (n, %)† | 24 (46.2%) | 45 (62.5%) | 0.07 |
| BUN (mg/dL) | 12.4±3.9 | 14.2±5.6 | 0.04 |
| >20 mg/dL (n, %)‡ | 1 (1.9%) | 12 (16.7%) | 0.01 |
| Creatinine (mg/dL) | 0.24±0.06 | 0.26±0.07 | 0.12 |
| AST (IU/L) | 48.8±34.7 | 46.1±10.2 | 0.53 |
| ALT (IU/L) | 31.5±39.7 | 24.9±9.2 | 0.17 |
| Na (mmol/L) | 136.8±2.6 | 135.9±3.3 | 0.11 |
| K (mmol/L) | 4.3±0.5 | 4.2±0.4 | 0.16 |
| Abnormal electrolytes (n, %)∫ | 9 (17.3%) | 23 (31.9%) | 0.07 |
| Total CO2 (mmol/L) | 15.9±3.1 | 14.6±3.1 | 0.02 |
| Urine specific gravity | 1.014±0.010 | 1.018±0.010 | 0.02 |
| Stool occult blood (ng/mL) | 11.5±73.3 | 5.4±18.0 | 0.50 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download