Abstract
Purpose
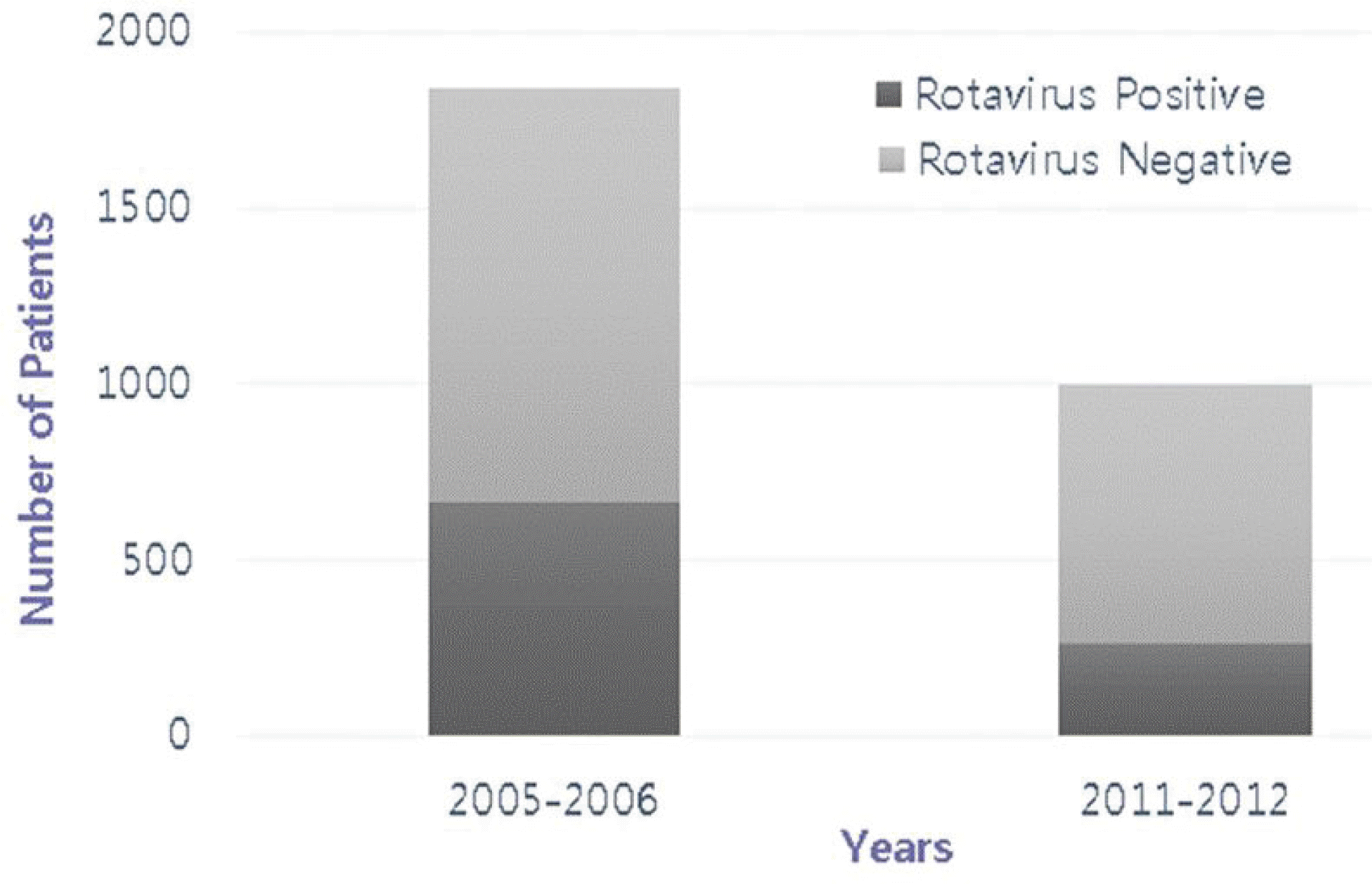
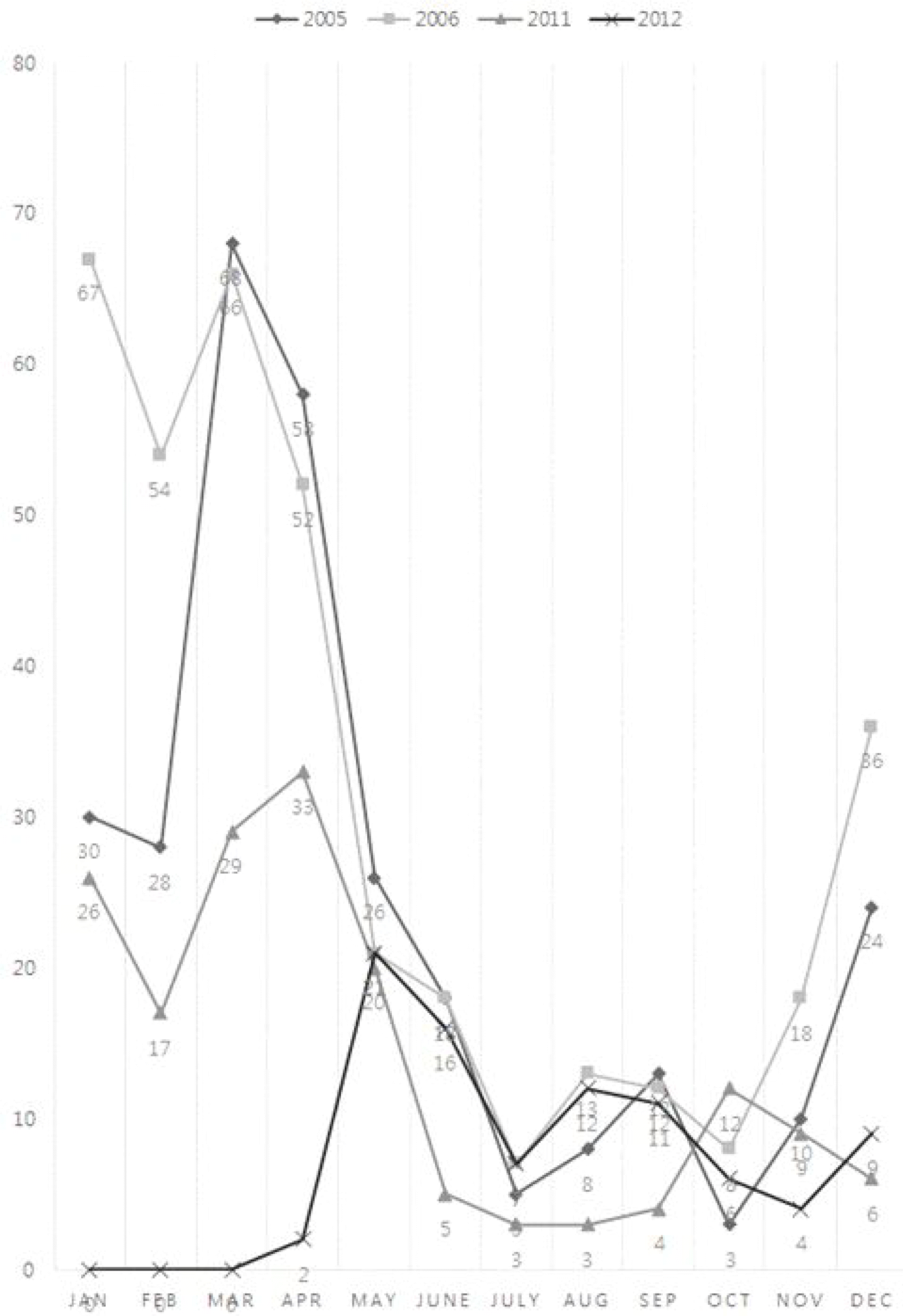
This study aimed to examine the changes in the outbreak of acute gastroenteritis, rotavirus gastroenteritis after the introduction of the rotavirus vaccine in Korea. Methods: The current study investigated the number of inpatients in the pediatric ward of Inje University Sanggye Paik Hospital during the periods of 2005-2006 and 2011-2012. A retrospective analysis was conducted on the medical records of 2,840 patients <5 years of age who were hospitalized at Inje University Sanggye Paik Hospital in these time periods. Results: When we compared 2 separate sets of data from before (2005–2006) and after (2011–2012) vaccine introduction, there were statistically significant decreases in the number of patients who were hospitalized for acute gastroenteritis across all of the groups of patients <5 years of age except those <2 months of age. The number of patients with rotavirus gastroenteritis in all age groups declined except for children <2 months of age and those 2–5 months of age. Conclusion: These results show that after the introduction of a rotavirus vaccine in Korea, the incidence of rotavirus gas-troenteritis decreased in 6–59-month-old patients hospitalized for acute gastroenteritis.
Go to : 
References
1. Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003; 9:565–72.

2. Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch Virol. 2011; 156:1397–413.

3. ThanVT. Kim W. Prevalence of rotavirus genotypes in South Korea in 1989–2009: implications for a nationwide rotavirus vaccine program. Korean J Pediatr. 2013; 56:465–73.

4. Kang JO, Kim MN, Kim J, Suh HS, Yoon Y, Jang S, et al. Epidemiologic trends of rotavirus infection in the Republic of Korea, July 1999 through June 2002. Korean J Lab Med. 2003; 23:382–7.
5. Jeong HS, Lee KB, Jeong AY, Jo MY, Jung SY, Ahn JH, et al. Genotypes of the circulating rotavirus strains in the seven prevaccine seasons from September 2000 to August 2007 in South Korea. Clin Microbiol Infect. 2011; 17:232–5.

6. Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006; 354:11–22.

7. Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomized, double-blind, placebocontrolled phase III study. Lancet. 2008; 371:1181–9.
8. Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriquez A, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006; 354:23–33.

9. Ruiz-Palacios GM, Pé rez-Schael I, Velá zquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006; 354:11–22.

10. Gastanaduy PA, Curns AT, Parashar UD, Lopman BA. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA. 2014; 310:851–3.

11. Desai R, Curns AT, Steiner CA, Tate JE, Patel MM, Parashar UD. All-cause gastroenteritis and rotaviuscoded hospitalizations among US children, 2000–2009. Clin Infect Dis. 2012; 55:e28–e34.
12. Yen C, Tate JE, Wenk JD, Harris JM, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics. 2011; 127:e9–e15.

13. do Carmo GM, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med. 2011; 8:e10011024.

14. Lappalainen S, Ylitalo S, Arola A, Halkosalo A, Rasanen S, Vesikari T. Simultaneous presence of human herpesvirus 6 and adenovirus infections in intestinal intussusception of young children. Acta Paediatr. 2012; 101:663–70.

15. Aminu M, Ameh EA, Geyer A, Esona MD, Taylor MB, Steele AD. Role of astrovirus in intussusception in Nigerian infants. J Trop Pediatr. 2009; 55:192–4.

16. Velazquez FR, Luna G, Cedillo R, Torres J, Munoz O. Natural rotavirus infection is not associated to intussusception in Mexican children. Pediatr Infect Dis. 2004; 23:S173–8.
17. Chang EJ, Zangwill KM, Lee H, Ward JI. Lack of asso-ciation between rotavirus infection and intussusception: implications for use of attenuated rotavirus vaccines. Pediatr Infect Dis J. 2002; 21:97–102.

18. Bahl R, Saxena M, Bhandari N, Taneja S, Mathur M, Parashar UD, et al. Population-based incidence of intussusception and a case-control study to examine the asso-ciation of intussusception with natural rotavirus infection among Indian children. J Infect Dis. 2009; 200:277–81.

19. Robinson CG, Hernanz-Schulman M, Zhu Y, Griffin MR, Gruber W, Edwards KM. Evaluation of anatomic changes in young children with natural rotavirus infection: is intussusception biologically plausible? J Infect Dis. 2004; 189:1382–7.

20. Belongia EA, Irving SA, Shui IM, Kulldorff M, Lewis E, Yin R, et al. Realtime surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Peidatr Infect Dis J. 2010; 29:1–5.

21. Shui IM, Baggs J, Patel M, Parashar UD, Rett M, Belongia EA, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA. 2012; 307:598–604.

22. Yih WK, Lieu TA, Kulldorff M, Martin D, McMahil-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014; 370:503–12.

23. Haber P, Patel M, Pan Y, Baggs J, Haber M, Museru O, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006–2012. Pediatrics. 2013; 131:1042–9.

24. Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliviera LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011; 364:2283–92.
25. Yen C, Tate JE, Steiner CA, Cortese MM, Patel MM, Parashar UD. Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000–2009. J Infect Dis. 2012; 206:41–8.

26. Desai R, Curns AT, Steiner CA, Tate JE, Patel MM, Parashar UD. All-cause gastroenteritis and rotavirus-coded hospitalizations among US children, 2000–2009. Clin Infect Dis. 2012; 55:e28–34.

27. Choi UY, Lee SY, Ma SH, Lee SY, Jang YT, Kim JY, at al. Epidemiological changes in rotavirus gastroenteritis in children under 5 years of age after the introduction of rotavirus vaccines in Korea. Eur J Pediatr. 2013; 172:947–52.
28. Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013. 1283–8.

29. Jacques LP, Kristina N, Nathalie RC. Host-pathogen co-evolution and glycan interactions. Curr Opin Virol. 2014; 7:88–94.
Go to : 
Table 1.
Acute Gastroenteritis-related Hospitalization and Rotavirus Gastroenteritis-related Hospitalization by Age Group
Table 2.
Rotavirus Vaccination Coverage among Ro-tavirus Negative Gastroenteritis
Table 3.
Rotavirus Vaccination Coverage among Ro-tavirus Positive Gastroenteritis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download