Abstract
Purpose
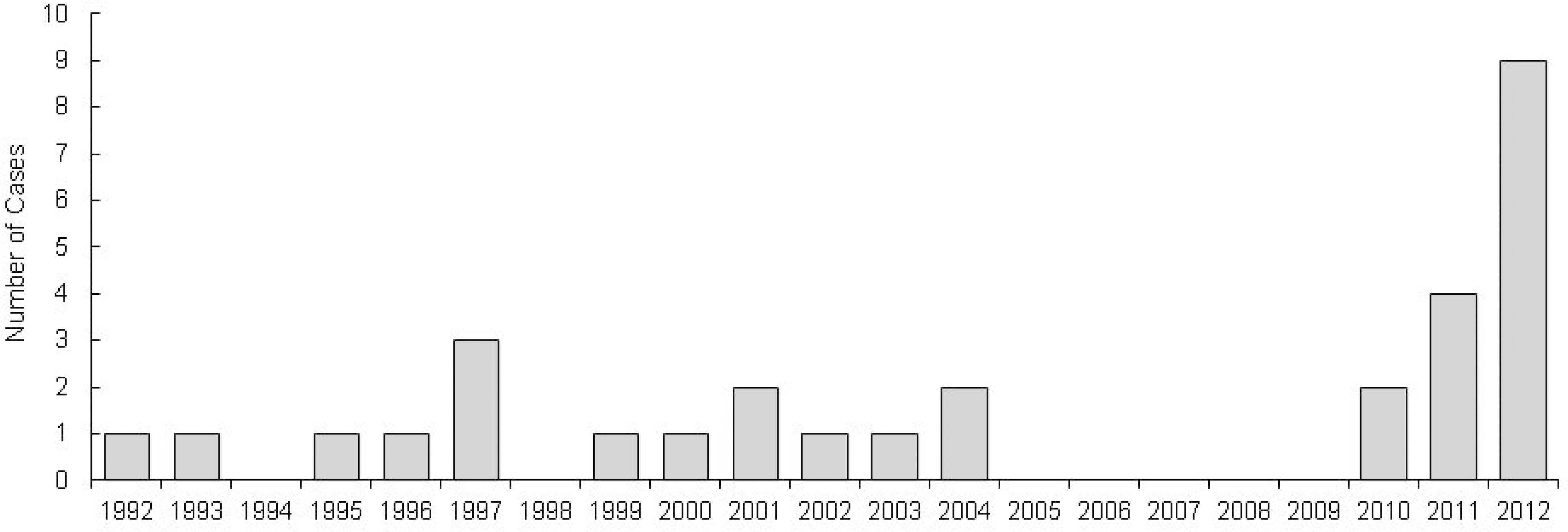
Streptococcus pyogenes is an important cause of invasive diseases in children. We aimed to describe the clinical characteristics of invasive infections due to S. pyogenes in children in Korea. Methods: A retrospective study of children under 18 years of age with invasive infections due to S. pyogenes at Seoul National University Children' s Hospital between March 1992 and December 2012, and Seoul National University Bundang Hospital between March 2003 and December 2012 was conducted. Demographic factors, clinical characteristics, laboratory findings, treatment, mortality and morbidity of all patients were reviewed. Results: A total of 30 among 36 cases identified as invasive disease due to S. pyogenes were available for review. There was a predominance for male subjects (male:female=2.75:1). The median age was 50 months (range 12 days to 15 years) and 53.3% were under 5 years of age. Skin and soft tissue infections (9/30, 30.0%), bacteremia without identified focus (4/30, 13.3%) and bone and joint infections (6/30, 20.0%) were the most frequent clinical presentations. Streptococcal toxic shock syndrome (3/30, 10.0%) pulmonary, abdomen and central nervous system infections (2/30, 6.7%) were also seen. There was a peak in number of patients in year 2012 (9/30, 30.0%). There were no cases of mortality. Erythromycin and clindamycin resistance rates were low by 3.8% and 7.5%, respectively. Conclusion: We studied the clinical presentations of invasive infections due to S. pyogenes during the past 20 years in Korean children. The findings of this study help us understand the characteristics of the disease, enhancing early recognition and prompting adequate antibiotic therapy which is important in reducing morbidity and mortality.
References
1. World Health Organization. The current evidence for the burden of group A streptococcal diseases. 2005.
2. Meehan M, Murchan S, Bergin S, O'Flanagan D, Cunney R. Increased incidence of invasive group A streptococcal disease in Ireland, 2012 to 2013. Euro Surveill. 2013; 18:20556.

3. Health Protection Report, 2013. Group A streptococcal infections: update on seasonal activity 2012/2013. Available from:. http://www.hpa.org.uk/hpr/archives/2013/news1713.htm.
4. Darenberg J, Henriques-Normark B, Lepp T, Tegmark-Wisell K, Tegnell A, Widgren K. Increased incidence of invasive group A streptococcal infections in Sweden, January 2012-February 2013. Euro Surveill. 2013; 18:20443.

5. Stockmann C, Ampofo K, Hersh AL, Blaschke AJ, Kendall BA, Korgenski K, et al. Evolving epidemiologic characteristics of invasive group a streptococcal disease in Utah, 2002–2010. Clin Infect Dis. 2012; 55:479–87.

6. Lamagni TL, Darenberg J, Luca-Harari B, Siljander T, Efstratiou A, Henriques-Normark B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008; 46:2359–67.
7. Minodier P, Bidet P, Rallu F, Tapiero B, Bingen E, Ovet-chkine P. Clinical and microbiologic characteristics of group A streptococcal necrotizing fasciitis in children. Pediatr Infect Dis J. 2009; 28:541–3.

8. Lepoutre A, Doloy A, Bidet P, Leblond A, Perrocheau A, Bingen E, et al. Epidemiology of invasive Streptococcus pyogenes infections in France in 2007. J Clin Microbiol. 2011; 49:4094–5100.

9. Lee JH, Cho HK, Kim KH, Kim CH, Kim DS, Kim KN, et al. Etiology of invasive bacterial infections in immunocompetent children in Korea (1996–2005): a retrospective multicenter study. J Korean Med Sci. 2011; 26:174–83.

10. Cho EY, Kim YJ, Eun BW, Kim YK, Cho DS, Lee HS, et al. Causative pathogens among childhood bacterial invasive infection in Korea; a multicenter study, 2006–2010. In: The 2012 Annual Meeting of the Korean Society of Pediatric Infectious Diseases;. 2012. Nov 10; Seoul, Korea.
11. Cho EY, Choi EH, Lee H, Kang JH, Kim KH, Lee HJ, et al. Multicentric approach for analysis of serotypes of pneumococcus isolated from invasive infections in Korean children. Korea. In: The 2013 Annual Meeting of the Korean Society of Pediatric Infectious Diseases;. 2013. Nov 9; Seoul, Korea.
12. Rodrí guez-Nuñ ez A, Dosil-Gallardo S, Jordan I. ad hoc Streptococcal toxic shock syndrome collaborative group of Spanish society of pediatric intensive care. Clinical characteristics of children with group A streptococcal toxic shock syndrome admitted to pediatric intensive care units. Eur J Pediatr. 2011; 170:639–44.
13. McMillan DJ, Drè ze PA, Vu T, Bessen DE, Guglielmini J, Steer AC. Updated model of group A streptococcus M proteins based on a comprehensive worldwide study. Clin Microbiol Infect. 2013; 19:E222–9.

14. Martin DR, Single LA. Molecular epidemiology of group A streptococcus M type 1 infections. J Infect Dis. 1993; 167:1112–7.

15. O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007; 45:853–62.
16. Tyrrell GJ, Lovgren M, Kress B, Grimsrud K. Invasive group A streptococcal disease in Alberta, Canada (2000 to 2002). J Clin Microbiol. 2005; 43:1678–83.

17. Kim SH. Epidemiology and erythromycin resistance of Streptococcus pyogenes in the last 20 years. Korean J Clin Microbiol. 2011; 14:119–125.
18. Fujita K, Murono K, Yoshikawa M, Murai T. Decline of erythromycin resistance of group A streptococci in Japan. Pediatr Infect Dis J. 1994; 13:1075–8.

19. Bass JW, Weisse ME, Plymyer MR, Murphy S, Eberly BJ. Decline of erythromycin resistance of group A betahemolytic streptococci in Japan. Comparison with world-wide reports. Arch Pediatr Adolesc Med. 1994; 148:67–71.
20. Bergman M, Huikko S, Pihlajamä ki M, Laippala P, Palva E, Huovinen P, et al. Finnish Study Group for Antimicrobial Resistance (FiRe Network). Effect of macrolide consumption on erythromycin resistance in Streptococcus pyogenes in Finland in 1997–2001.Clin Infect Dis. 2004; 38:1251–6.
21. Koh E, Kim S. Decline in erythromycin resistance in group A streptococci from acute pharyngitis due to changes in the emm genotypes rather than restriction of antibiotic use. Korean J Lab Med. 2010; 30:485–90.

22. Oliver MA, Garcí a-Delafuente C, Cano ME, Pé rez-Herná ndez F, Martí nez-Martí nez L, Albertí S. Rapid decrease in the prevalence of macrolide-resistant group A streptococci due to the appearance of two epidemic clones in Cantabria (Spain). J Antimicrob Chemother. 2007; 60:450–2.

23. American Academy of Pediatrics. Committee on Infectious Diseases. Severe invasive group A streptococcal infections: a subject review. Pediatrics. 1998; 101:136–40.
24. Wilson GJ, Talkington DF, Gruber W, Edwards K, Der-mody TS. Group A streptococcal necrotizing fasciitis following varicella in children: case reports and review. Clin Infect Dis. 1995; 20:1333–8.

25. Doctor A, Harper MB, Fleisher GR. Group A betahemolytic streptococcal bacteremia: historical overview, changing incidence, and recent association with varicella. Pediatrics. 1995; 96:428–33.
Table 1.
Clinical Presentation of 30 Patients with In-vasive Infection Caused by Streptococcus pyogenes,1992–2012
| Clinical symptom and sign | No. of patients (%) |
|---|---|
| Fever | 29 (96.6) |
| Exanthema | 18 (60) |
| Respiratory symptoms | 13 (43.3) |
| (cough, rhinorrhea, respiratory distress) | |
| Soft tissue inflammation | 9 (30) |
| (swelling, erythema, tenderness) | |
| Vomiting, abdominal pain, diarrhea | 7 (23.3) |
| Sore throat | 6 (20) |
| Sensorium alteration | 6 (20) |
| (somnolence, irritability) | |
| Arthralgia | 6 (20) |
| Skin wound∗ | 3 (10) |
Table 2.
Clinical Diagnosis of 30 Episodes of Invasive Infection Caused by Streptococcus pyogenes, 1992–2012
| Diagnosis∗ | No. of patients (%) |
|---|---|
| Bacteremia without focus | 4† (13.3) |
| Skin and soft tissue infection | 9 (30.0) |
| Cellulitis | 3‡ |
| Omphalitis | 2 |
| Myositis | 1 |
| Deep neck infection | 1 |
| Lymphadenitis | 1 |
| Lymphangioma infection | 1 |
| Osteoarticular infection | 6∫ (20.0) |
| Septic arthritis | 5∥ |
| Osteomyelitis | 3 |
| Streptococcal toxic shock syndrome | 3 (10.0) |
| Pulmonary infection | 2 (6.7) |
| Pneumonia | 2 |
| Abdomen infection | 2 (6.7) |
| Peritonitis | 1 |
| Cholangitis | 1 |
| Central nervous system infection | 2 (6.7) |
| Meningitis | 1 |
| Subdural empyema | 1 |
| Infective endocarditis | 2 (6.7) |
| Total | 30 (100) |
Table 3.
Clinical Characteristics of 30 Episodes of Invasive Infection Caused by Streptococcus pyogenes, 1992–2012
| Characteristics n (%) or median [range] | SSTI | Osteoarticular infection | Bacteremia Without focus | STSS | Pulmonary infection | CNS infection | Abdomen infection | Infective endocarditis |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| N (overall %) | 9 (30) | 6 (20) | 4 (13.3) | 3(10) | 2 (6.6) | 2 (6.6) | 2 (6.6) | 2 (6.6) |
| Age | 4.7 | 6.9 | 3.7 | 4.4 | 3.4 | 14.3 | 9.5 | 4.4 |
| Sex(M/F) | 5/4 | 5/1 | 3/1 | 3/0 | 1/1 | 2/0 | 2/0 | 1/1 |
| Underlying condition N (% within group) | ||||||||
| Underlying disease | 2 (22.2) | 0 (0) | 3 (75) | 1 (33.3) | 1 (50) | 0 (0) | 2 (100) | 1 (50) |
| Varicella infection | 0 (0) | 0 (0) | 1 (25) | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cutaneous wound | 3 (33.3) | 0 (0) | 0 (0) | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Minor trauma | 1 (11.1) | 1 (16.6) | 0 (0) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Treatment Duration of | 17.4 | 32.8 | 11.5 | 29.3 | 11 | 40.5 | 29 | 36 |
| antibiotics (days) | [9–30] | [17–45] | [10–15] | [21–34] | [6–16] | [25–56] | [22–36] | [30–42] |
| Duration of IV | 12.5 | 20.5 | 5.2 | 27 | 6 | 30 | 29 | 36 |
| antibiotics (days) | [4–21] | [14–29] | [1–10] | [21–33] | [18–42] | [22–36] | [30–42] | |
| Surgery | 2 | 6 | 0 | 2 | 0 | 1 | 0 | 1 |
| Mechanical ventilation | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 0 |
| Immunoglobulin | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 |
| Outcome Duration of | 2.6 | 5 | 6.5 | 22 | 3.5 | 8.5 | 3.5 | 9.5 |
| fever (days) | [0–9] | [2–10] | [4–12] | [12–35] | [2–5] | [5–12] | [2–5] | [2–17] |
| ICU care | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 1 |
| Duration of | 6 | 0 | 0 | 23.6 | 6 | 3 | 0 | 15 |
| ICU stay (days) | [10–31] | |||||||
| Hospitalization | 12.6 | 18.6 | 8 | 61.6 | 6.5 | 31 | 30.5 | 38.5 |
| (days) | [5–17] | [5–31] | [2–11] | [34–104] | [19–43] | [22–39] | [31–46] | |
| Sequelae | 0 | 0 | 0 | 2† | 1‡ | 2∫ | 0 | 0 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviation: SSTI, skin and soft tissue infection; STSS, streptococcal toxic shock syndrome; CNS, central nervous system.∗Cases were defined as when Streptococcus pyogenes was isolated from a normally sterile site or specimen, including blood, ascites, joint aspiration, cerebrospinal fluid or operation specimens.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download