Abstract
Purpose :
To assess the immunogenicity, safety, and tolerability of hepatitis A vaccine (VAQTATM) in healthy children and adolescents.
Methods :
Eligible subjects aged 2 to 17 years received 25 U/0.5 mL of VAQTATM intramuscularly at 0 and 24 week schedule. Bleeds were obtained prior to vaccination and 4 weeks after the second dose to ascertain serostatus. To detect antibody to HAV after vaccination with an inactivated HA vaccine, a modification of the Abbott® HAVAB kit was used. Sample with titers > 10 mIU/mL were considered seroconverted. Adverse experiences were monitored.
Results :
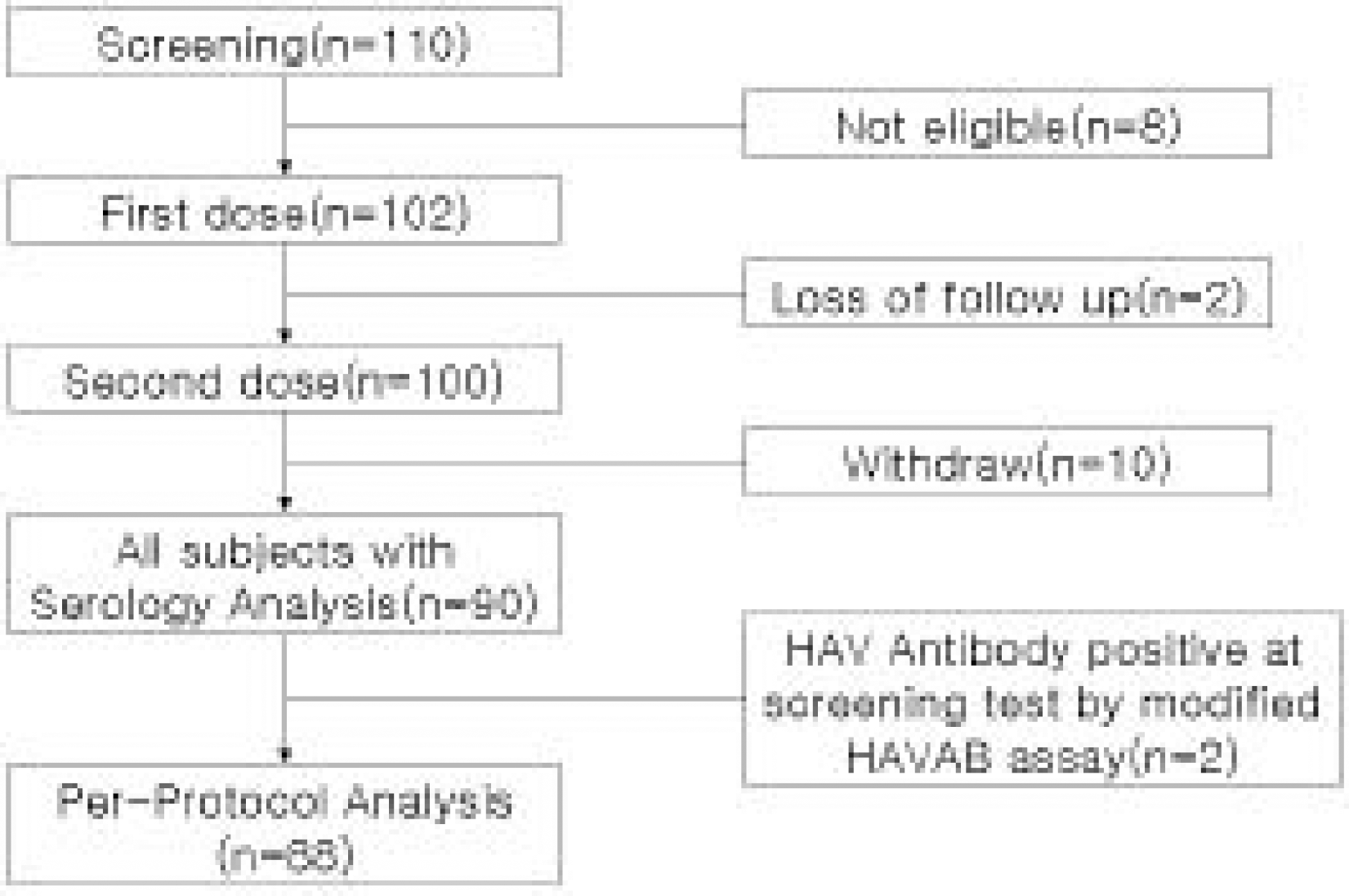
102 subjects(54 male, 48 female) were enrolled. The mean age was 6.8±3.5 years. Two subjects were seropositive, two were lost of follow up. 88 subjects were available for a per protocol analysis and 90 for all subjects with serology after the second dose, and ten withdral. All subjects(95% CI, 94.8〜 100) seroconverted. Geometric mean titers was 7,991.1(95% CI, 6,481.1〜9,852.7) with very little difference in per protocol analysis and all subjects analysis. Adverse experiences to VAQTATM were generally mild and transient.
Conclusion :
The pediatric two-dose regimen of VAQTATM was found to be highly im- munogenic, generally well tolerated and resulted in 100% seroconversion. Regarding Korea is in transition from a high to low risk region resulting in a paradox increase of clinical disease and disease burden, routine vaccination should be considered in order to control hepatitis A effectively.
Go to : 
Table 1.
Sero posit iv ity Rate and Geometric Mean Titers
| Week 28 | SPR | GMT(titer) |
|---|---|---|
| PP(n=88) | 100%(94.8~100.0) | 7,991.1(6,481.2~9,852.7) mlU/mL |
| AS(n=90) | 100%(94.9~100.0) | 7,906.4(6,437.2~9,710.8) mlU/mL |
Table 2.
Summary of Adverse Experiences
Table 3.
Number(%) of Reported Vaccine-related Adverse Experiences (AE) by 14 Days after Inject ion




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download