Abstract
Purpose
2009 Pandemic influenza A (H1N1) virus was identified in March 2009 and subsequently caused worldwide outbreaks. We described the clinical and epidemiological characteristics of H1N1 influenza infection.
Methods
We used retrospective medical chart reviews to collect data on the visiting patients from a single institute. H1N1 infection was confirmed in specimens with the use of a RT-PCR (real time reverse transcriptase polymerase chain reaction assay).
Result
6,836 patients had H1N1 RT-PCR test, and 2,781 were confirmed with H1N1 virus infection. 158 patients (5.7%) had ± ± hospital treatment and inpatients were significantly younger (5.4 3.3 years) than outpatients (7.5 3.9 years) among H1N1 virus confirmed patients. Oxygen, steroid, immunoglobulin, ventilator treatment was provided in a substantial proportion among pneumonia patients accompanying wheezy respiration. In addition more intensive care was needed in patients accompanying segmental, lobar, interstitial, mixed pneumonia and lung effusion (27.2%) than patients with bronchopneumonia (7.3%) among H1N1 virus infection confirmed patients. Seventy-one infants had oseltamivir treatment out of 83 infants under 1 year, and no significant side effects and complications were identified.
Conclusion
In 2009 pandemic influenza A (H1N1), hospital treatment was needed in younger patients. Early intensive care was needed in pneumonia patients accompanying wheezy respiration, and patients accompanying segmental, lobar, inter-stitial, mixed pneumonia and lung effusion. (Korean J Pediatr Infect Dis 2011;18:173–181)
Go to : 
References
1). Centers for Disease Control and Prevention (CDC). Outbreak of swine origin influenza A (H1N1) virus infection-Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:467–70.
2). Influenza A (H1N1)-update 44. Geneva: WHO;2009. Available at:. http://www.who.int/csr/don/2009_06_05/en/index.html. Accessed 6 June 2009.
3). CDC. CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April – December 12, 2009. Available at:. http://www.cdc.gov/h1n1flu/estimates/April_December_12.htm.
4). Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009; 360:2605–15.
5). Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Griffin J, Hollingsworth TD, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009; 324:1557–61.

6). Turbelin C, Pelat C, Bo lle PY, L vy-Bruhl D, Carrat ë é F, Blanchon T, et al. Early estimates of 2009 pandemic influenza A(H1N1) virus activity in general practice in France: incidence of influenza-like illness and age distribution of reported cases. Euro Surveill. 2009; 14:19341.

7). Garske T, Legrand J, Donnelly CA, Ward H, Cauchemez S, Fraser C, et al. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ. 2009; 339:b2840.

8). Korea Center for Disease Control and Prevention (KCDC). Epidemiology of early detected Novel Influenza A (H1N1) in Korea, 2009. Public Health Wkly Rep. 2009; 2:689–91.
9). Garten RJ, Davis CT, Russell CA, Shu B, Lindstorm S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science. 2009; 325:197–201.
10). Loftus BG, Price JF. Clinical and immunological characteristics of pre-school asthma. Clin Allergy. 1986; 16:251–7.

11). Peiris J, Poon L, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. Journal of Clinical Virology. 2009; 45:169–73.

12). Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009; 459:931–9.

13). Seong HW, Woo JK. Diagnosis and management of novel influenza A(H1N1). Korean J Fam Med. 2009; 30:843–7.
14). Public Health Agency of Canada. Flu watch: July 5, 2009 to July 11, 2009 (week 27). http://www.phac-aspc.gc.ca/fluwatch/08–09/w27_09/index-eng.php. Accessed July 20,. 2009.
15). Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998; 178:53–60.

16). Hui DS, Lee N, Chan PK. Clinical management of pandemic 2009 influenza A (H1N1) infection. Chest. 2010; 137:916–25.
17). Kim SH. Treatment of severe pandemic influenza A/H1N1 Infection. Infect Chemother. 2009; 5:265–71.

18). Faix DJ, Sherman SS, Waterman SH. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009; 361:728–9.

19). Reddy D. Responding to pandemic (H1N1) 2009 influenza: the role of oseltamivir. J Antimicrob Chemother. 2010; 65(Suppl 2):ii35–40.

20). CDC. Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection – United States, April-August 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:941–7.
22). Lee HJ, Min SJ, Choi JW, Kang EK. Clinical characteristics of hospitalized pediatric patients with 2009 novel influenza A infection. Pediatr Allergy Dis (Korea). 2010; 20:130–7.
23). Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Qui oses-Falcon F, Bautista E, et al. ñ Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Eng J Med. 2009; 361:680–9.
24). Mollura DJ, Aspis DS, Conetta R, Feign DS, Bray M, Taubenberger JK, et al. Imaging findings in a fatal case of pandemic swine-origin influenza A (H1N1). AJR Am J Roentgenol. 2009; 193:1500–3.

25). Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009; 193:1488–93.

26). Jain S, Kamimoto L, Bramley AM, Schmitz AM, Louie J, Sugerman DE, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Eng J Med. 2009; 361:1935–44.

27). Quispe-Laime AM, Bracco JD, Barberio PA, Campagne CG, Rolfo VE, Umberger R, et al. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010; 36:33–41.

28). Cunha BA. Swine Influenza (H1N1) Pneumonia: Clinical Considerations. Infect Dis Cli Am. 2009; 24:203–28.

29). Dawood FS, Kamimoto L, D'Mello TA, Reingold A, Gershman K, Meek J. Children with asthma hospitalized with seasonal or pandemic influenza, 2003–2009. Pediatrics. 2011; 12:e27–32. Epub 2011 Jun 6.

30). Smith JR, Ariano RE, Toovey S. The use of antiviral agents for the management severe influenza. Cric Care Med. 2010; 38:e43–51.
31). Okamoto S, Kamiya I, Kishida K, Shimakawa T, Fukui T, Morimoto T. Experience with oseltamivir for infants younger than 1 year old in Japan. Pediatr Infect Dis J. 2005; 24:575–6.

32). Tamura D, Miura T, Kikuchi Y. Oseltamivir phosphate in infants under 1 year of age with influenza infection. Pediatr Int. 2005; 47:484.

33). CDC Seasonal Flu Website. Recommendations for Use of Antiviral Medications for the Management of Influenza in Children and Adolescent for the 2009–2010 Season – Pediatric Supplement for Health Care Providers. Available at:. http://www.cdc.gov/h1n1flu/recommendations_pediatric_supplement.htm.
Go to : 
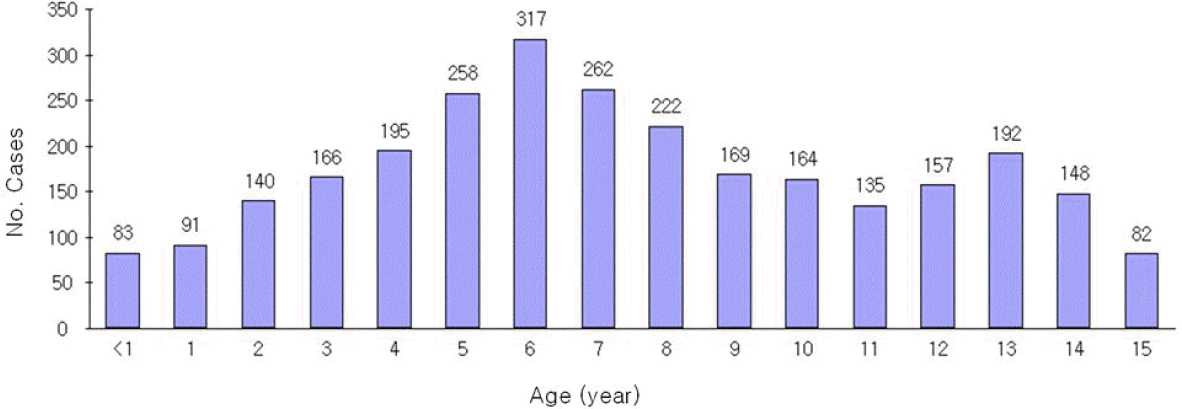 | Fig. 2.Age-specific incidence cases for pandemic influenza A (H1N1) 2009 infection in a single Institute. |
Table 1.
Characteristics of the Inpatients and Outpatients Who Had Confirmed Infection Pandemic Influenza A(H1N1) 2009
| Inpatient (N=158) | Outpatient (N=2,623) | Total (N=2,781) | P-value | |
|---|---|---|---|---|
| Age | 5.4±3.3 | 7.5±3.9 | 7.4±3.9 | <0.001 |
| M/F | M: 104 (65.8) | M: 1,537 (58.5) | M:1,641 (59.0) | 0.073 |
| F: 54 (34.2) | F: 1,086 (41.5) | F:1,140 (41.0) | ||
| Symptom & Sign | ||||
| Fever No. (%) | 155 (98.1) | 2,574 (98.1) | 2,730 (98.1) | 0.95 |
| Cough No. (%) | 140 (88.6) | 2,276 (88.6) | 2,416 (86.8) | 0.507 |
| Rhinorrea No. (%) | 107 (67.7) | 1,454 (55.4) | 1,561 (56.1) | 0.003 |
| Sore throat No. (%) | 8 (5.0) | 909 (34.6) | 917 (32.9) | <0.001 |
| headache No. (%) | 8 (5.0) | 760 (28.9) | 768 (27.6) | <0.001 |
| Generalache No. (%) | 6 (3.7) | 297 (11.3) | 303 (10.8) | 0.003 |
| Vomiting No. (%) | 11 (6.9) | 289 (11.0) | 300 (10.7) | 0.110 |
| Nausea No. (%) | 3 (1.8) | 126 (4.8) | 129 (4.6) | 0.092 |
| wheezing No. (%) | 57 (36.0) | 12 (0.4) | 69 (2.4) | <0.001 |
| Dyspnea No. (%) | 20 (12.6) | 0 (0) | 20 (0.7) | <0.001 |
| Diarrhea No. (%) | 3 (1.8) | 15 (0.5) | 18 (0.6) | 0.043 |
| Laboratory findings | Inpatient (N=158) | Outpatient (N=123)† | Total (N=281) | P-value |
| WBC (/mm3) | 8,651.1±4,324.0 | 7,602.3±3,069.2 | 8,141.4±3,849.1 | 0.038 |
| ESR (mm/Hr) | 11.2±10.9 | 7.6±7.5 | 9.6±9.9 | 0.001 |
| CRP (mg/dL) | 3.1±3.8 | 1.1±1.3 | 2.2±3.1 | <0.001 |
| Lymphopenia (%) | 20.3±17.4 | 20.9±12.8 | 20.6±15.5 | 0.76 |
| Lymphocyte (/mm3) | 1,479.8±1,362.0 | 1517.3±1082.6 | 1496.0±1252.1 | 0.389 |
Table 2.
Laboratory Findings of Bronchopneumonia and Other Pneumonia for the Diagnosis of 2009 Influenza A (H1N1) Virus Infection
Table 3.
Comparison between Pneumonia Patients and Pneumonia Patients Accompanying Wheezy Respiraton for the Diagnosis of 2009 Influenza A (H1N1) Virus Infection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download